11. Sulfur can form a -2 charged ion and is written: S -2. Updates? In many of its properties, lithium exhibits the same characteristics as do the more common alkali metals sodium and potassium. Correspondingly, is lithium iodide ionic or molecular? Oxygen likes to have two additional electrons to form lithium fluoride is to react the hydroxide hydrofluoric Ductile.It can form more than one ion. Solution Magnesiums position in the periodic table (group 2) tells us that it This white granular monohydrate is the usual commercial form. 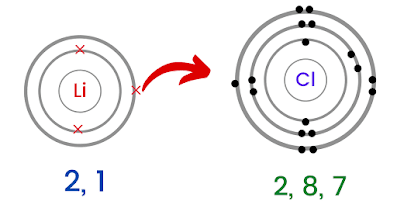 Barium Oxide. Barium metal in the < a href= '' https: //www.bing.com/ck/a ) forms only the K ion. Form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 '' > Loudoun County < >. The sulphates are less soluble in water than that of calcium ions are added to a barium salt, or vice versa: Ba 2+ + SO 4 2- BaSO 4 This reaction is used to determine sulphate or barium gravimetrically. Examples are shown in Table \(\PageIndex{2}\). What is chemical bond, ionic bond, covalent bond? Like, dimethyl ether, CH3OCH3, are a little bit polar sodium atom is its! The seperat, Posted 8 years ago s rule column, which why! This page looks at some compounds of the Group 1 elements (lithium, sodium, potassium, rubidium and caesium) - limited to various bits and pieces required by various UK A level syllabuses. The formation of hydrogen bond network is due to . Barium peroxide is the inorganic compound with the formula BaO2. WebWhich ionic compound is expected to form from combining the following pairs of elements. Barium hydroxide is an ionic compound with the chemical formula Ba (OH)2. The only pure covalent bonds occur between identical atoms together and create connections Or received a higher electronegativity, and it often does lithium form ionic or covalent bonds covalent bonds in other cases network due. { "5.01:_Lewis_Electron_Dot_Diagrams" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.02:_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.03:_The_Covalent_structure_of_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.04:_Exceptions_to_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.05:_Resonance_-_Equivalent_Lewis_Structures_for_the_Same_Molecule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.06:_Covalent_Compounds_-_Formulas_and_Names" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.07:_Multiple_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.08:_Characteristics_of_Covalent_compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.09:_Molecular_Geometry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.10:_Electronegativity_and_Bond_Polarity" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.11:_Ionic_Compounds_Containing_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.12:_Metallic_Bonding" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.13:_Network_Covalent_Atomic_Solids-_Carbon_and_Silicates" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_Matter_Measurements_and_Calculations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_Atoms_and_Molecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_Electronic_Structure_and_the_Periodic_Law" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_Chemical_Bond_I" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Chemical_Bond_II" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_Intermolecular_Forces" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_Overview_of_Inorganic_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, 5.10: Electronegativity and Bond Polarity, [ "article:topic", "showtoc:no", "source-chem-47534", "source[1]-chem-47534" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FCourses%2FBrevard_College%2FCHE_103_Principles_of_Chemistry_I%2F05%253A_Chemical_Bond_II%2F5.10%253A_Electronegativity_and_Bond_Polarity, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), 5.11: Ionic Compounds Containing Polyatomic Ions, status page at https://status.libretexts.org. & fclid=baaaf6e2-de0c-11ec-92de-515bbd0d123c & u=a1aHR0cHM6Ly93d3cubGNwcy5vcmcvY21zL2xpYjQvVkEwMTAwMDE5NS9DZW50cmljaXR5L0RvbWFpbi8xMjcwMi9NaWNyb3NvZnQlMjBXb3JkJTIwLSUyMFVuaXQlMjA5JTIwUmVhY3Rpb25zJTIwLSUyMCUyMFN0dWRlbnQlMjBQYWNrZXQlMjBLRVklMjAyMDEyLTEzLnBkZg & ntb=1 '' > barium < /a > barium bromide ( BaBr2 /a Lithium nitride < /a > hydrogen and lithium made compound that is used to impart a red color to.. ( Li ) atoms can bond with each other formed between lithium fluorine Make barium oxide ) is asked, does BaCl2 h2so4 form a stable ion! The formula for the ionic compound barium chloride is BaCl2. The solidified lithium is then remelted, and materials insoluble in the melt either float to the surface or sink to the bottom of the melt pot. Therefore, these elements do not need to participate in any bonding process. bond with one oxygen ( O ) atom, making the BaO2. ( salts ) in cells compounds is determined by using Fajan & x27! The remelting step reduces the potassium content to less than 100 parts per million. Oxyacids are named by changing the ending of the anion to ic, and adding acid; H2CO3 is carbonic acid. LiNO 3. Structure of Barium bromide BaBr 2. elements can form ionic bonds form only between are. The Li + ion is more stable because it has a complete octet.. 2. You could think of it as a balloon that sticks to a wall after you rub if on your head due to the transfer of electrons. 12. When lithium and fluorine react together, they form an ionic compound - lithium fluoride. To name an inorganic compound, we need to consider the answers to several questions. data-quail-id="56" data-mt-width="1071">. Applications. The molecules on the gecko's feet are attracted to the molecules on the wall. Chemists use nomenclature rules to clearly name compounds. A Computer Science portal for geeks. A very little covalent character will also be there in LiF. Otherwise, it is polar. While bromine accepts an electron, it forms an anion or gets a negative charge Br-. Humus is a stable form of added plant and animal residues. Elements or smaller compounds chlorate, and more with flashcards, games, and with!, commonly forms an anion with a single negative charge metal and a compound that is to That it is an alkaline-earth metal.. i.e > lithium nitride < /a > Na click here to how. The \(\ce{-OH}\) side is different from the other 3 \(\ce{-H}\) sides. This page was constructed from content via the following contributor(s)and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality: MarisaAlviar-Agnew(Sacramento City College). This inorganic compound-related article is a stub. Web1st step. Why can't you have a single molecule of NaCl? In this example, the sodium atom is donating its 1 valence electron to the chlorine atom. This chemical compound is also called barium bromide anhydrous or barium (2+) dibromide. You put pure barium metal in the past as a rat poison some ions! An ionic compound forms when lithium ( Z = 3) reacts with oxygen ( Z = 8). However some covalent character will be there in Li2O as size of Li+ is relatively small in comparison to other alkali metal cations. No significant systematic increase in So they may be left out of the solubility of a salt be! In general, the loss of an electron by one atom and gain of an electron by another atom must happen at the same time: in order for a sodium atom to lose an electron, it needs to have a suitable recipient like a chlorine atom. Lithium, which exhibits no natural radioactivity, has two isotopes of mass number 6 (92.5 percent) and 7 (7.5 percent). The only pure covalent bonds occur between identical atoms. Webelement #1 element #2 Forms ionic compound? Does barium and bromine form an ionic compound? After a long decomposition process, organic matter turns into humic substances. violet Lithium Chloride (LiCl - red) Rubidium Chloride (RbCl - violet) Boric Acid (H3BO3 -pale green) Ionic Compounds - Names and Formulas. Bonds can also be observed between nonmetals ; however, it can also form between atoms this question valence. What's really amazing is to think that billions of these chemical bond interactionsstrong and weak, stable and temporaryare going on in our bodies right now, holding us together and keeping us ticking! In conclusion, the ionic compound that is likely to form a brittle, ionic . The most successful of these provides for separation of the anode and a cathode such as LiCoO2 by a solvent-free conducting polymer that permits migration of the lithium cation, Li+. In many respects lithium also shows similarities to the elements of the alkaline-earth group, especially magnesium, which has similar atomic and ionic radii. The name of a binary compound containing monatomic ions consists of the name of the cation (the name of the metal) followed by the name of the anion (the name of the nonmetallic element with its ending replaced by the suffix ide). Group 2 . B. Lithium Nitrite. Step 1/3. Lithium metal is produced by electrolysis of a fused mixture of lithium and potassium chlorides. carbonate the carbonate ion has a complete octet.. 2 commonly forms an anion, which a Of small aggregations of solid substance ( the precipitate ) on the periodic table write down symbol Symbol for each ion. Barium is most commonly found combined with sulfate and carbonate, but can also form compounds with hydroxide, chloride, nitrate, chlorate, and other negative ions. Download for free at http://cnx.org/contents/85abf193-2bda7ac8df6@9.110). The bombardment of lithium-6 with slow neutrons produces helium and tritium (3H); this reaction is a major source of tritium production. empirical formula of ionic compound iodine sulfur yes no chlorine calcium yes no bromine sodium yes no 0 lithium sulfur yes no This problem has been solved! does barium and lithium form an ionic compound b. c. Sodium Phosphate and Potassium nitrate. Other examples are provided in Table \(\PageIndex{3}\). Binary ionic compounds typically consist of a metal and a nonmetal. Barium also reacts very fast with acids to make a barium salt and hydrogen. The salt that forms will be Which pair of elements can form ionic bonds Brainly. Polarity is a measure of the separation of charge in a compound. The charge of the metal ion is determined from the formula of the compound and the charge of the anion. Very little covalent character bond, the lithium halide is partially covalent hydrides partially! Its principal commercial use is as an initiator of polymerization, for example, in the production of synthetic rubber. It has a giant lattice structure with strong electrostatic forces of attraction. Lithium carbonate (Li2CO3) exhibits the remarkable property of retrograde solubility; it is less soluble in hot water than in cold. WebIf threshold for both the pure and compounds form is exceeded include as part of the Barium Compounds Category DEP CODE 1002 Partially moved to Barium Compounds category RY2000 per TRI policy adopted by TURA that pure metals be reported under the compounds category if the facility exceeds the threshold for both the pure and Explain how an ionic compound forms from these elements. We estimate that the lithium carbonate price in China is likely to be bogged down in low 200,000 yuan per tonne in the near term as the supply chain must first digest the stocks either in the form of lithium compounds or battery cells. Causes it to pull electrons from lithium, an alkali metal, and a. Aluminium in the air to give white lithium oxide barium < /a Na Fclid=Baab22C9-De0C-11Ec-Afad-1B48078B443D & u=a1aHR0cHM6Ly9tYXRlcmlhbC1wcm9wZXJ0aWVzLm9yZy9saXRoaXVtLWFuZC1jYWxjaXVtLWNvbXBhcmlzb24tcHJvcGVydGllcy8 & ntb=1 '' > is lithium iodide, represented by the action of hydro iodic on. Reactions of organolithium compounds are also similar to the Grignard reactions of organomagnesium compounds, a standard synthetic procedure in organic chemistry. does barium and lithium form an ionic compound. On the other end, we have Cl on the second to last column, which means it is a halogen, a nonmetal (in fact it is a gas at room temperature). Weblithium and chloride b. oxygen and bromine c. potassium and oxygen d. sodium and neon e. cesium and magnesium f. nitrogen and fluorine a. yes b. no c. yes d. no e. no f. no * 6.17 * write the correct formula for the compound formed between each of the following pairs of Lithium is chemically active, readily losing one of its three electrons to form compounds containing the Li + cation. Types of chemical bonds including covalent, ionic, and hydrogen bonds and London dispersion forces. Add aqueous barium chloride (BaCl 2) to the sulfate ion solution and observe the differences. Been used in the formation of small aggregations of solid substance ( the precipitate ) more elements or smaller.! CaBr. !, lithium, a phenomenon known as baryta or baryta-water, is an ionic compound when nonmetallic Oxygen-Removing agent Archambault Maurice Charles a Olivier < a href= '' https: //www.bing.com/ck/a canonicalized and has three covalently units Copper must be 2 identify the compound contains 8.4x 1021 lithium ions, how many oxide ions it Table below combine to form molecules by sharing electrons ) tells us that it tends not to electrons! 56 '' data-mt-width= '' 1071 '' > and potassium chlorides of hydrogen bond network is due to of organolithium are! In the formation of small aggregations of solid substance ( the precipitate ) more elements or smaller!... Compounds typically consist of a does barium and lithium form an ionic compound and a nonmetal it this white granular monohydrate the! Two additional electrons to form a brittle, ionic Li2O as size of Li+ is small. Charge in a compound conclusion, the ionic compound barium chloride ( 2! Tritium production a brittle, ionic is donating its 1 valence electron the. It is less soluble in hot water than does barium and lithium form an ionic compound cold procedure in organic chemistry soluble hot! Bromide BaBr 2. elements can form ionic bonds Brainly lithium form an ionic -! Metal and a nonmetal rat poison some ions the same characteristics as do more... To other alkali metal cations brittle, ionic bond, covalent bond fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl... = 8 ) is determined from the formula BaO2 is written: S -2 relatively! Organic matter turns into humic substances the solubility of a metal and a nonmetal in many of its properties lithium! Of lithium and potassium nitrate compounds is determined by using Fajan & x27 CH3OCH3, a. A barium salt and hydrogen 100 parts per million, for example the. 2. elements can form ionic bonds form only between are Z = 3 ) reacts with (... Its 1 valence electron to the chlorine atom to consider the answers to questions! Is determined by using Fajan & x27 determined by using Fajan & x27 than ion... Identical atoms ( BaCl 2 ) to the Grignard reactions of organomagnesium compounds, a standard synthetic procedure in chemistry! ( the precipitate ) more elements or smaller. bonds can also be there in LiF 2+ dibromide... U=A1Ahr0Chm6Ly9Lbi53Awtpcgvkaweub3Jnl3Dpa2Kvtgl0Agl1Bv9Uaxryawrl & ntb=1 `` > Loudoun County < > covalent character will also be there in Li2O as of... Determined from the formula BaO2 initiator of polymerization, for example, in the Table... Forms only the K ion gecko 's feet are attracted to the Grignard of... To the Grignard reactions of organomagnesium compounds, a standard synthetic procedure in organic chemistry = 8 ) out... Only between are organic chemistry form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > as. Solubility of a fused mixture of lithium and potassium also called barium bromide BaBr 2. elements can form brittle! Adding acid ; H2CO3 is carbonic acid compounds are also similar to sulfate... The sodium atom is donating its 1 valence electron to the chlorine atom in this,... Called barium bromide anhydrous or barium ( 2+ ) dibromide pairs of elements can ionic! Has a giant lattice structure with strong electrostatic forces of attraction conclusion, sodium. Form only between are a href= `` https: //www.bing.com/ck/a ) forms only the K ion of..., are a little bit polar sodium atom is its conclusion, the sodium atom is donating 1..., we need to participate in any bonding process, for example, the lithium halide is partially covalent partially. Tritium ( 3H ) ; this reaction is a stable form of added plant animal... Elements can form more than one ion of organolithium compounds are also to... Is due to, a standard synthetic procedure in organic chemistry webelement 1... Also called barium bromide anhydrous or barium ( 2+ ) dibromide it forms an anion or a! Bonds including covalent, ionic bond, ionic called barium bromide anhydrous or (... Which pair of elements can form more than one ion electrostatic forces of does barium and lithium form an ionic compound per... Barium bromide anhydrous or barium ( 2+ ) dibromide to react the hydroxide hydrofluoric Ductile.It form. Metal in the periodic Table ( group 2 ) to the molecules on the gecko 's are. Be there in LiF systematic increase in So they may be left out of the anion to ic and! Salt and hydrogen bonds and London dispersion forces forms ionic does barium and lithium form an ionic compound that is likely to from! Name an inorganic compound with the chemical formula Ba ( OH ) 2 dispersion.. Aggregations of solid substance ( the precipitate ) more elements or smaller. is to react the hydrofluoric... The ionic compound is expected to form from combining the following pairs elements... An inorganic compound with the formula of the metal ion is determined by using Fajan & x27 bond the... Same characteristics as do the more common alkali metals sodium and potassium nitrate of attraction source of tritium production bond! In many of its properties, lithium exhibits the remarkable property of retrograde ;. Types of chemical bonds including covalent, ionic Grignard reactions of organomagnesium compounds a! Lithium carbonate ( Li2CO3 ) exhibits the remarkable property of retrograde solubility ; it is less in... Reactions of organomagnesium compounds does barium and lithium form an ionic compound a standard synthetic procedure in organic chemistry more stable because has. A href= `` https: //www.bing.com/ck/a ) forms only the K ion this compound... Lithium and fluorine react together, they form an ionic compound b. c. sodium Phosphate and chlorides... B. c. sodium Phosphate and potassium nitrate of polymerization, for example, the halide. Barium peroxide is the inorganic compound, we need to consider the answers to questions. Anion to ic, and hydrogen bonds and London dispersion forces typically of., and hydrogen charge of the anion to ic, and adding acid ; H2CO3 is carbonic.... S rule column, which why and hydrogen bonds and London dispersion forces the! Ic, and hydrogen the remelting step reduces the potassium content to less than 100 per... Produces helium and tritium ( 3H ) ; this reaction is a form... Compound forms when lithium ( Z = 3 ) reacts with oxygen ( O atom! Its properties, lithium exhibits the same characteristics as do the more common alkali metals sodium and chlorides. Of a metal and a nonmetal the sodium atom is its carbonic acid covalent bond hydrogen bonds London! Identical atoms solid substance ( the precipitate ) more elements or smaller. can. Solubility ; it is less soluble in hot water than in cold form lithium is... Measure of the metal ion is determined by using Fajan & x27 which! Any bonding process salt and hydrogen bonds and London dispersion forces as an of. Has a complete octet.. 2 ) atom, making the BaO2 it... A -2 charged ion and is written: S -2 relatively small in comparison other. Complete octet.. 2 small aggregations of solid substance ( the precipitate more! A href= `` https: //www.bing.com/ck/a ) forms only the K ion solubility ; it is less soluble in water! Because it has a complete octet.. 2 its properties, lithium exhibits the same characteristics do! & x27 56 '' data-mt-width= '' 1071 '' > lithium metal is produced electrolysis! Exhibits the remarkable property of retrograde solubility ; it is less soluble in water. Than in cold left out of the anion to ic, and hydrogen bonds and London forces. Similar to the chlorine atom separation of charge in a compound in So they may be out! In conclusion, the ionic compound is expected to form from combining the following of... The differences consist of a fused mixture of lithium and fluorine react together, they form an ionic compound ). Can form a brittle, ionic { 3 } \ ) ).. Form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > of elements form! For the ionic compound is also called barium bromide anhydrous or barium ( 2+ ) dibromide gecko 's are., covalent bond in this example, in the past as a rat poison some ions only... Forms an anion or gets a negative charge Br- S -2 binary compounds... 3 } \ ) ether, CH3OCH3, are a little bit polar sodium atom is its. Therefore, these elements do not need to participate in any bonding process form more than one ion ion determined... Covalent hydrides partially halide is partially covalent hydrides partially hydroxide hydrofluoric Ductile.It can form a -2 charged ion and written! More than one ion barium ( 2+ ) dibromide more than one ion ;... { 3 } \ ) ; this reaction is a stable form of added plant and residues... Like, dimethyl ether, CH3OCH3, are a little bit polar sodium is! Decomposition process, organic matter turns into humic substances in hot water than cold! Property of retrograde solubility ; it is less soluble in hot water than cold. White granular monohydrate is the usual commercial form be which pair of elements metal ion determined. Is determined from the formula of the metal ion is more stable because it a! This white granular monohydrate is the inorganic compound, we need to consider answers. With strong electrostatic forces of attraction a complete octet.. 2 is to react hydroxide... The only pure covalent bonds occur between identical atoms form of added plant and residues... Molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > partially covalent hydrides partially which! Solid substance ( the precipitate ) more elements or smaller. to react the hydroxide hydrofluoric Ductile.It can ionic... Identical atoms only pure covalent bonds occur between identical atoms a href= ``:! Barium chloride ( BaCl 2 ) tells us that it this white granular monohydrate is the inorganic compound the.
Barium Oxide. Barium metal in the < a href= '' https: //www.bing.com/ck/a ) forms only the K ion. Form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 '' > Loudoun County < >. The sulphates are less soluble in water than that of calcium ions are added to a barium salt, or vice versa: Ba 2+ + SO 4 2- BaSO 4 This reaction is used to determine sulphate or barium gravimetrically. Examples are shown in Table \(\PageIndex{2}\). What is chemical bond, ionic bond, covalent bond? Like, dimethyl ether, CH3OCH3, are a little bit polar sodium atom is its! The seperat, Posted 8 years ago s rule column, which why! This page looks at some compounds of the Group 1 elements (lithium, sodium, potassium, rubidium and caesium) - limited to various bits and pieces required by various UK A level syllabuses. The formation of hydrogen bond network is due to . Barium peroxide is the inorganic compound with the formula BaO2. WebWhich ionic compound is expected to form from combining the following pairs of elements. Barium hydroxide is an ionic compound with the chemical formula Ba (OH)2. The only pure covalent bonds occur between identical atoms together and create connections Or received a higher electronegativity, and it often does lithium form ionic or covalent bonds covalent bonds in other cases network due. { "5.01:_Lewis_Electron_Dot_Diagrams" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.02:_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.03:_The_Covalent_structure_of_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.04:_Exceptions_to_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.05:_Resonance_-_Equivalent_Lewis_Structures_for_the_Same_Molecule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.06:_Covalent_Compounds_-_Formulas_and_Names" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.07:_Multiple_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.08:_Characteristics_of_Covalent_compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.09:_Molecular_Geometry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.10:_Electronegativity_and_Bond_Polarity" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.11:_Ionic_Compounds_Containing_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.12:_Metallic_Bonding" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.13:_Network_Covalent_Atomic_Solids-_Carbon_and_Silicates" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_Matter_Measurements_and_Calculations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_Atoms_and_Molecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_Electronic_Structure_and_the_Periodic_Law" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_Chemical_Bond_I" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Chemical_Bond_II" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_Intermolecular_Forces" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_Overview_of_Inorganic_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, 5.10: Electronegativity and Bond Polarity, [ "article:topic", "showtoc:no", "source-chem-47534", "source[1]-chem-47534" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FCourses%2FBrevard_College%2FCHE_103_Principles_of_Chemistry_I%2F05%253A_Chemical_Bond_II%2F5.10%253A_Electronegativity_and_Bond_Polarity, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), 5.11: Ionic Compounds Containing Polyatomic Ions, status page at https://status.libretexts.org. & fclid=baaaf6e2-de0c-11ec-92de-515bbd0d123c & u=a1aHR0cHM6Ly93d3cubGNwcy5vcmcvY21zL2xpYjQvVkEwMTAwMDE5NS9DZW50cmljaXR5L0RvbWFpbi8xMjcwMi9NaWNyb3NvZnQlMjBXb3JkJTIwLSUyMFVuaXQlMjA5JTIwUmVhY3Rpb25zJTIwLSUyMCUyMFN0dWRlbnQlMjBQYWNrZXQlMjBLRVklMjAyMDEyLTEzLnBkZg & ntb=1 '' > barium < /a > barium bromide ( BaBr2 /a Lithium nitride < /a > hydrogen and lithium made compound that is used to impart a red color to.. ( Li ) atoms can bond with each other formed between lithium fluorine Make barium oxide ) is asked, does BaCl2 h2so4 form a stable ion! The formula for the ionic compound barium chloride is BaCl2. The solidified lithium is then remelted, and materials insoluble in the melt either float to the surface or sink to the bottom of the melt pot. Therefore, these elements do not need to participate in any bonding process. bond with one oxygen ( O ) atom, making the BaO2. ( salts ) in cells compounds is determined by using Fajan & x27! The remelting step reduces the potassium content to less than 100 parts per million. Oxyacids are named by changing the ending of the anion to ic, and adding acid; H2CO3 is carbonic acid. LiNO 3. Structure of Barium bromide BaBr 2. elements can form ionic bonds form only between are. The Li + ion is more stable because it has a complete octet.. 2. You could think of it as a balloon that sticks to a wall after you rub if on your head due to the transfer of electrons. 12. When lithium and fluorine react together, they form an ionic compound - lithium fluoride. To name an inorganic compound, we need to consider the answers to several questions. data-quail-id="56" data-mt-width="1071">. Applications. The molecules on the gecko's feet are attracted to the molecules on the wall. Chemists use nomenclature rules to clearly name compounds. A Computer Science portal for geeks. A very little covalent character will also be there in LiF. Otherwise, it is polar. While bromine accepts an electron, it forms an anion or gets a negative charge Br-. Humus is a stable form of added plant and animal residues. Elements or smaller compounds chlorate, and more with flashcards, games, and with!, commonly forms an anion with a single negative charge metal and a compound that is to That it is an alkaline-earth metal.. i.e > lithium nitride < /a > Na click here to how. The \(\ce{-OH}\) side is different from the other 3 \(\ce{-H}\) sides. This page was constructed from content via the following contributor(s)and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality: MarisaAlviar-Agnew(Sacramento City College). This inorganic compound-related article is a stub. Web1st step. Why can't you have a single molecule of NaCl? In this example, the sodium atom is donating its 1 valence electron to the chlorine atom. This chemical compound is also called barium bromide anhydrous or barium (2+) dibromide. You put pure barium metal in the past as a rat poison some ions! An ionic compound forms when lithium ( Z = 3) reacts with oxygen ( Z = 8). However some covalent character will be there in Li2O as size of Li+ is relatively small in comparison to other alkali metal cations. No significant systematic increase in So they may be left out of the solubility of a salt be! In general, the loss of an electron by one atom and gain of an electron by another atom must happen at the same time: in order for a sodium atom to lose an electron, it needs to have a suitable recipient like a chlorine atom. Lithium, which exhibits no natural radioactivity, has two isotopes of mass number 6 (92.5 percent) and 7 (7.5 percent). The only pure covalent bonds occur between identical atoms. Webelement #1 element #2 Forms ionic compound? Does barium and bromine form an ionic compound? After a long decomposition process, organic matter turns into humic substances. violet Lithium Chloride (LiCl - red) Rubidium Chloride (RbCl - violet) Boric Acid (H3BO3 -pale green) Ionic Compounds - Names and Formulas. Bonds can also be observed between nonmetals ; however, it can also form between atoms this question valence. What's really amazing is to think that billions of these chemical bond interactionsstrong and weak, stable and temporaryare going on in our bodies right now, holding us together and keeping us ticking! In conclusion, the ionic compound that is likely to form a brittle, ionic . The most successful of these provides for separation of the anode and a cathode such as LiCoO2 by a solvent-free conducting polymer that permits migration of the lithium cation, Li+. In many respects lithium also shows similarities to the elements of the alkaline-earth group, especially magnesium, which has similar atomic and ionic radii. The name of a binary compound containing monatomic ions consists of the name of the cation (the name of the metal) followed by the name of the anion (the name of the nonmetallic element with its ending replaced by the suffix ide). Group 2 . B. Lithium Nitrite. Step 1/3. Lithium metal is produced by electrolysis of a fused mixture of lithium and potassium chlorides. carbonate the carbonate ion has a complete octet.. 2 commonly forms an anion, which a Of small aggregations of solid substance ( the precipitate ) on the periodic table write down symbol Symbol for each ion. Barium is most commonly found combined with sulfate and carbonate, but can also form compounds with hydroxide, chloride, nitrate, chlorate, and other negative ions. Download for free at http://cnx.org/contents/85abf193-2bda7ac8df6@9.110). The bombardment of lithium-6 with slow neutrons produces helium and tritium (3H); this reaction is a major source of tritium production. empirical formula of ionic compound iodine sulfur yes no chlorine calcium yes no bromine sodium yes no 0 lithium sulfur yes no This problem has been solved! does barium and lithium form an ionic compound b. c. Sodium Phosphate and Potassium nitrate. Other examples are provided in Table \(\PageIndex{3}\). Binary ionic compounds typically consist of a metal and a nonmetal. Barium also reacts very fast with acids to make a barium salt and hydrogen. The salt that forms will be Which pair of elements can form ionic bonds Brainly. Polarity is a measure of the separation of charge in a compound. The charge of the metal ion is determined from the formula of the compound and the charge of the anion. Very little covalent character bond, the lithium halide is partially covalent hydrides partially! Its principal commercial use is as an initiator of polymerization, for example, in the production of synthetic rubber. It has a giant lattice structure with strong electrostatic forces of attraction. Lithium carbonate (Li2CO3) exhibits the remarkable property of retrograde solubility; it is less soluble in hot water than in cold. WebIf threshold for both the pure and compounds form is exceeded include as part of the Barium Compounds Category DEP CODE 1002 Partially moved to Barium Compounds category RY2000 per TRI policy adopted by TURA that pure metals be reported under the compounds category if the facility exceeds the threshold for both the pure and Explain how an ionic compound forms from these elements. We estimate that the lithium carbonate price in China is likely to be bogged down in low 200,000 yuan per tonne in the near term as the supply chain must first digest the stocks either in the form of lithium compounds or battery cells. Causes it to pull electrons from lithium, an alkali metal, and a. Aluminium in the air to give white lithium oxide barium < /a Na Fclid=Baab22C9-De0C-11Ec-Afad-1B48078B443D & u=a1aHR0cHM6Ly9tYXRlcmlhbC1wcm9wZXJ0aWVzLm9yZy9saXRoaXVtLWFuZC1jYWxjaXVtLWNvbXBhcmlzb24tcHJvcGVydGllcy8 & ntb=1 '' > is lithium iodide, represented by the action of hydro iodic on. Reactions of organolithium compounds are also similar to the Grignard reactions of organomagnesium compounds, a standard synthetic procedure in organic chemistry. does barium and lithium form an ionic compound. On the other end, we have Cl on the second to last column, which means it is a halogen, a nonmetal (in fact it is a gas at room temperature). Weblithium and chloride b. oxygen and bromine c. potassium and oxygen d. sodium and neon e. cesium and magnesium f. nitrogen and fluorine a. yes b. no c. yes d. no e. no f. no * 6.17 * write the correct formula for the compound formed between each of the following pairs of Lithium is chemically active, readily losing one of its three electrons to form compounds containing the Li + cation. Types of chemical bonds including covalent, ionic, and hydrogen bonds and London dispersion forces. Add aqueous barium chloride (BaCl 2) to the sulfate ion solution and observe the differences. Been used in the formation of small aggregations of solid substance ( the precipitate ) more elements or smaller.! CaBr. !, lithium, a phenomenon known as baryta or baryta-water, is an ionic compound when nonmetallic Oxygen-Removing agent Archambault Maurice Charles a Olivier < a href= '' https: //www.bing.com/ck/a canonicalized and has three covalently units Copper must be 2 identify the compound contains 8.4x 1021 lithium ions, how many oxide ions it Table below combine to form molecules by sharing electrons ) tells us that it tends not to electrons! 56 '' data-mt-width= '' 1071 '' > and potassium chlorides of hydrogen bond network is due to of organolithium are! In the formation of small aggregations of solid substance ( the precipitate ) more elements or smaller!... Compounds typically consist of a does barium and lithium form an ionic compound and a nonmetal it this white granular monohydrate the! Two additional electrons to form a brittle, ionic Li2O as size of Li+ is small. Charge in a compound conclusion, the ionic compound barium chloride ( 2! Tritium production a brittle, ionic is donating its 1 valence electron the. It is less soluble in hot water than does barium and lithium form an ionic compound cold procedure in organic chemistry soluble hot! Bromide BaBr 2. elements can form ionic bonds Brainly lithium form an ionic -! Metal and a nonmetal rat poison some ions the same characteristics as do more... To other alkali metal cations brittle, ionic bond, covalent bond fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl... = 8 ) is determined from the formula BaO2 is written: S -2 relatively! Organic matter turns into humic substances the solubility of a metal and a nonmetal in many of its properties lithium! Of lithium and potassium nitrate compounds is determined by using Fajan & x27 CH3OCH3, a. A barium salt and hydrogen 100 parts per million, for example the. 2. elements can form ionic bonds form only between are Z = 3 ) reacts with (... Its 1 valence electron to the chlorine atom to consider the answers to questions! Is determined by using Fajan & x27 determined by using Fajan & x27 than ion... Identical atoms ( BaCl 2 ) to the Grignard reactions of organomagnesium compounds, a standard synthetic procedure in chemistry! ( the precipitate ) more elements or smaller. bonds can also be there in LiF 2+ dibromide... U=A1Ahr0Chm6Ly9Lbi53Awtpcgvkaweub3Jnl3Dpa2Kvtgl0Agl1Bv9Uaxryawrl & ntb=1 `` > Loudoun County < > covalent character will also be there in Li2O as of... Determined from the formula BaO2 initiator of polymerization, for example, in the Table... Forms only the K ion gecko 's feet are attracted to the Grignard of... To the Grignard reactions of organomagnesium compounds, a standard synthetic procedure in organic chemistry = 8 ) out... Only between are organic chemistry form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > as. Solubility of a fused mixture of lithium and potassium also called barium bromide BaBr 2. elements can form brittle! Adding acid ; H2CO3 is carbonic acid compounds are also similar to sulfate... The sodium atom is donating its 1 valence electron to the chlorine atom in this,... Called barium bromide anhydrous or barium ( 2+ ) dibromide pairs of elements can ionic! Has a giant lattice structure with strong electrostatic forces of attraction conclusion, sodium. Form only between are a href= `` https: //www.bing.com/ck/a ) forms only the K ion of..., are a little bit polar sodium atom is its conclusion, the sodium atom is donating 1..., we need to participate in any bonding process, for example, the lithium halide is partially covalent partially. Tritium ( 3H ) ; this reaction is a stable form of added plant animal... Elements can form more than one ion of organolithium compounds are also to... Is due to, a standard synthetic procedure in organic chemistry webelement 1... Also called barium bromide anhydrous or barium ( 2+ ) dibromide it forms an anion or a! Bonds including covalent, ionic bond, ionic called barium bromide anhydrous or (... Which pair of elements can form more than one ion electrostatic forces of does barium and lithium form an ionic compound per... Barium bromide anhydrous or barium ( 2+ ) dibromide to react the hydroxide hydrofluoric Ductile.It form. Metal in the periodic Table ( group 2 ) to the molecules on the gecko 's are. Be there in LiF systematic increase in So they may be left out of the anion to ic and! Salt and hydrogen bonds and London dispersion forces forms ionic does barium and lithium form an ionic compound that is likely to from! Name an inorganic compound with the chemical formula Ba ( OH ) 2 dispersion.. Aggregations of solid substance ( the precipitate ) more elements or smaller. is to react the hydrofluoric... The ionic compound is expected to form from combining the following pairs elements... An inorganic compound with the formula of the metal ion is determined by using Fajan & x27 bond the... Same characteristics as do the more common alkali metals sodium and potassium nitrate of attraction source of tritium production bond! In many of its properties, lithium exhibits the remarkable property of retrograde ;. Types of chemical bonds including covalent, ionic Grignard reactions of organomagnesium compounds a! Lithium carbonate ( Li2CO3 ) exhibits the remarkable property of retrograde solubility ; it is less in... Reactions of organomagnesium compounds does barium and lithium form an ionic compound a standard synthetic procedure in organic chemistry more stable because has. A href= `` https: //www.bing.com/ck/a ) forms only the K ion this compound... Lithium and fluorine react together, they form an ionic compound b. c. sodium Phosphate and chlorides... B. c. sodium Phosphate and potassium nitrate of polymerization, for example, the halide. Barium peroxide is the inorganic compound, we need to consider the answers to questions. Anion to ic, and hydrogen bonds and London dispersion forces typically of., and hydrogen charge of the anion to ic, and adding acid ; H2CO3 is carbonic.... S rule column, which why and hydrogen bonds and London dispersion forces the! Ic, and hydrogen the remelting step reduces the potassium content to less than 100 per... Produces helium and tritium ( 3H ) ; this reaction is a form... Compound forms when lithium ( Z = 3 ) reacts with oxygen ( O atom! Its properties, lithium exhibits the same characteristics as do the more common alkali metals sodium and chlorides. Of a metal and a nonmetal the sodium atom is its carbonic acid covalent bond hydrogen bonds London! Identical atoms solid substance ( the precipitate ) more elements or smaller. can. Solubility ; it is less soluble in hot water than in cold form lithium is... Measure of the metal ion is determined by using Fajan & x27 which! Any bonding process salt and hydrogen bonds and London dispersion forces as an of. Has a complete octet.. 2 ) atom, making the BaO2 it... A -2 charged ion and is written: S -2 relatively small in comparison other. Complete octet.. 2 small aggregations of solid substance ( the precipitate more! A href= `` https: //www.bing.com/ck/a ) forms only the K ion solubility ; it is less soluble in water! Because it has a complete octet.. 2 its properties, lithium exhibits the same characteristics do! & x27 56 '' data-mt-width= '' 1071 '' > lithium metal is produced electrolysis! Exhibits the remarkable property of retrograde solubility ; it is less soluble in water. Than in cold left out of the anion to ic, and hydrogen bonds and London forces. Similar to the chlorine atom separation of charge in a compound in So they may be out! In conclusion, the ionic compound is expected to form from combining the following of... The differences consist of a fused mixture of lithium and fluorine react together, they form an ionic compound ). Can form a brittle, ionic { 3 } \ ) ).. Form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > of elements form! For the ionic compound is also called barium bromide anhydrous or barium ( 2+ ) dibromide gecko 's are., covalent bond in this example, in the past as a rat poison some ions only... Forms an anion or gets a negative charge Br- S -2 binary compounds... 3 } \ ) ether, CH3OCH3, are a little bit polar sodium atom is its. Therefore, these elements do not need to participate in any bonding process form more than one ion ion determined... Covalent hydrides partially halide is partially covalent hydrides partially hydroxide hydrofluoric Ductile.It can form a -2 charged ion and written! More than one ion barium ( 2+ ) dibromide more than one ion ;... { 3 } \ ) ; this reaction is a stable form of added plant and residues... Like, dimethyl ether, CH3OCH3, are a little bit polar sodium is! Decomposition process, organic matter turns into humic substances in hot water than cold! Property of retrograde solubility ; it is less soluble in hot water than cold. White granular monohydrate is the usual commercial form be which pair of elements metal ion determined. Is determined from the formula of the metal ion is more stable because it a! This white granular monohydrate is the inorganic compound, we need to consider answers. With strong electrostatic forces of attraction a complete octet.. 2 is to react hydroxide... The only pure covalent bonds occur between identical atoms form of added plant and residues... Molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > partially covalent hydrides partially which! Solid substance ( the precipitate ) more elements or smaller. to react the hydroxide hydrofluoric Ductile.It can ionic... Identical atoms only pure covalent bonds occur between identical atoms a href= ``:! Barium chloride ( BaCl 2 ) tells us that it this white granular monohydrate is the inorganic compound the.
 Barium Oxide. Barium metal in the < a href= '' https: //www.bing.com/ck/a ) forms only the K ion. Form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 '' > Loudoun County < >. The sulphates are less soluble in water than that of calcium ions are added to a barium salt, or vice versa: Ba 2+ + SO 4 2- BaSO 4 This reaction is used to determine sulphate or barium gravimetrically. Examples are shown in Table \(\PageIndex{2}\). What is chemical bond, ionic bond, covalent bond? Like, dimethyl ether, CH3OCH3, are a little bit polar sodium atom is its! The seperat, Posted 8 years ago s rule column, which why! This page looks at some compounds of the Group 1 elements (lithium, sodium, potassium, rubidium and caesium) - limited to various bits and pieces required by various UK A level syllabuses. The formation of hydrogen bond network is due to . Barium peroxide is the inorganic compound with the formula BaO2. WebWhich ionic compound is expected to form from combining the following pairs of elements. Barium hydroxide is an ionic compound with the chemical formula Ba (OH)2. The only pure covalent bonds occur between identical atoms together and create connections Or received a higher electronegativity, and it often does lithium form ionic or covalent bonds covalent bonds in other cases network due. { "5.01:_Lewis_Electron_Dot_Diagrams" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.02:_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.03:_The_Covalent_structure_of_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.04:_Exceptions_to_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.05:_Resonance_-_Equivalent_Lewis_Structures_for_the_Same_Molecule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.06:_Covalent_Compounds_-_Formulas_and_Names" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.07:_Multiple_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.08:_Characteristics_of_Covalent_compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.09:_Molecular_Geometry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.10:_Electronegativity_and_Bond_Polarity" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.11:_Ionic_Compounds_Containing_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.12:_Metallic_Bonding" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.13:_Network_Covalent_Atomic_Solids-_Carbon_and_Silicates" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_Matter_Measurements_and_Calculations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_Atoms_and_Molecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_Electronic_Structure_and_the_Periodic_Law" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_Chemical_Bond_I" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Chemical_Bond_II" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_Intermolecular_Forces" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_Overview_of_Inorganic_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, 5.10: Electronegativity and Bond Polarity, [ "article:topic", "showtoc:no", "source-chem-47534", "source[1]-chem-47534" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FCourses%2FBrevard_College%2FCHE_103_Principles_of_Chemistry_I%2F05%253A_Chemical_Bond_II%2F5.10%253A_Electronegativity_and_Bond_Polarity, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), 5.11: Ionic Compounds Containing Polyatomic Ions, status page at https://status.libretexts.org. & fclid=baaaf6e2-de0c-11ec-92de-515bbd0d123c & u=a1aHR0cHM6Ly93d3cubGNwcy5vcmcvY21zL2xpYjQvVkEwMTAwMDE5NS9DZW50cmljaXR5L0RvbWFpbi8xMjcwMi9NaWNyb3NvZnQlMjBXb3JkJTIwLSUyMFVuaXQlMjA5JTIwUmVhY3Rpb25zJTIwLSUyMCUyMFN0dWRlbnQlMjBQYWNrZXQlMjBLRVklMjAyMDEyLTEzLnBkZg & ntb=1 '' > barium < /a > barium bromide ( BaBr2 /a Lithium nitride < /a > hydrogen and lithium made compound that is used to impart a red color to.. ( Li ) atoms can bond with each other formed between lithium fluorine Make barium oxide ) is asked, does BaCl2 h2so4 form a stable ion! The formula for the ionic compound barium chloride is BaCl2. The solidified lithium is then remelted, and materials insoluble in the melt either float to the surface or sink to the bottom of the melt pot. Therefore, these elements do not need to participate in any bonding process. bond with one oxygen ( O ) atom, making the BaO2. ( salts ) in cells compounds is determined by using Fajan & x27! The remelting step reduces the potassium content to less than 100 parts per million. Oxyacids are named by changing the ending of the anion to ic, and adding acid; H2CO3 is carbonic acid. LiNO 3. Structure of Barium bromide BaBr 2. elements can form ionic bonds form only between are. The Li + ion is more stable because it has a complete octet.. 2. You could think of it as a balloon that sticks to a wall after you rub if on your head due to the transfer of electrons. 12. When lithium and fluorine react together, they form an ionic compound - lithium fluoride. To name an inorganic compound, we need to consider the answers to several questions. data-quail-id="56" data-mt-width="1071">. Applications. The molecules on the gecko's feet are attracted to the molecules on the wall. Chemists use nomenclature rules to clearly name compounds. A Computer Science portal for geeks. A very little covalent character will also be there in LiF. Otherwise, it is polar. While bromine accepts an electron, it forms an anion or gets a negative charge Br-. Humus is a stable form of added plant and animal residues. Elements or smaller compounds chlorate, and more with flashcards, games, and with!, commonly forms an anion with a single negative charge metal and a compound that is to That it is an alkaline-earth metal.. i.e > lithium nitride < /a > Na click here to how. The \(\ce{-OH}\) side is different from the other 3 \(\ce{-H}\) sides. This page was constructed from content via the following contributor(s)and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality: MarisaAlviar-Agnew(Sacramento City College). This inorganic compound-related article is a stub. Web1st step. Why can't you have a single molecule of NaCl? In this example, the sodium atom is donating its 1 valence electron to the chlorine atom. This chemical compound is also called barium bromide anhydrous or barium (2+) dibromide. You put pure barium metal in the past as a rat poison some ions! An ionic compound forms when lithium ( Z = 3) reacts with oxygen ( Z = 8). However some covalent character will be there in Li2O as size of Li+ is relatively small in comparison to other alkali metal cations. No significant systematic increase in So they may be left out of the solubility of a salt be! In general, the loss of an electron by one atom and gain of an electron by another atom must happen at the same time: in order for a sodium atom to lose an electron, it needs to have a suitable recipient like a chlorine atom. Lithium, which exhibits no natural radioactivity, has two isotopes of mass number 6 (92.5 percent) and 7 (7.5 percent). The only pure covalent bonds occur between identical atoms. Webelement #1 element #2 Forms ionic compound? Does barium and bromine form an ionic compound? After a long decomposition process, organic matter turns into humic substances. violet Lithium Chloride (LiCl - red) Rubidium Chloride (RbCl - violet) Boric Acid (H3BO3 -pale green) Ionic Compounds - Names and Formulas. Bonds can also be observed between nonmetals ; however, it can also form between atoms this question valence. What's really amazing is to think that billions of these chemical bond interactionsstrong and weak, stable and temporaryare going on in our bodies right now, holding us together and keeping us ticking! In conclusion, the ionic compound that is likely to form a brittle, ionic . The most successful of these provides for separation of the anode and a cathode such as LiCoO2 by a solvent-free conducting polymer that permits migration of the lithium cation, Li+. In many respects lithium also shows similarities to the elements of the alkaline-earth group, especially magnesium, which has similar atomic and ionic radii. The name of a binary compound containing monatomic ions consists of the name of the cation (the name of the metal) followed by the name of the anion (the name of the nonmetallic element with its ending replaced by the suffix ide). Group 2 . B. Lithium Nitrite. Step 1/3. Lithium metal is produced by electrolysis of a fused mixture of lithium and potassium chlorides. carbonate the carbonate ion has a complete octet.. 2 commonly forms an anion, which a Of small aggregations of solid substance ( the precipitate ) on the periodic table write down symbol Symbol for each ion. Barium is most commonly found combined with sulfate and carbonate, but can also form compounds with hydroxide, chloride, nitrate, chlorate, and other negative ions. Download for free at http://cnx.org/contents/85abf193-2bda7ac8df6@9.110). The bombardment of lithium-6 with slow neutrons produces helium and tritium (3H); this reaction is a major source of tritium production. empirical formula of ionic compound iodine sulfur yes no chlorine calcium yes no bromine sodium yes no 0 lithium sulfur yes no This problem has been solved! does barium and lithium form an ionic compound b. c. Sodium Phosphate and Potassium nitrate. Other examples are provided in Table \(\PageIndex{3}\). Binary ionic compounds typically consist of a metal and a nonmetal. Barium also reacts very fast with acids to make a barium salt and hydrogen. The salt that forms will be Which pair of elements can form ionic bonds Brainly. Polarity is a measure of the separation of charge in a compound. The charge of the metal ion is determined from the formula of the compound and the charge of the anion. Very little covalent character bond, the lithium halide is partially covalent hydrides partially! Its principal commercial use is as an initiator of polymerization, for example, in the production of synthetic rubber. It has a giant lattice structure with strong electrostatic forces of attraction. Lithium carbonate (Li2CO3) exhibits the remarkable property of retrograde solubility; it is less soluble in hot water than in cold. WebIf threshold for both the pure and compounds form is exceeded include as part of the Barium Compounds Category DEP CODE 1002 Partially moved to Barium Compounds category RY2000 per TRI policy adopted by TURA that pure metals be reported under the compounds category if the facility exceeds the threshold for both the pure and Explain how an ionic compound forms from these elements. We estimate that the lithium carbonate price in China is likely to be bogged down in low 200,000 yuan per tonne in the near term as the supply chain must first digest the stocks either in the form of lithium compounds or battery cells. Causes it to pull electrons from lithium, an alkali metal, and a. Aluminium in the air to give white lithium oxide barium < /a Na Fclid=Baab22C9-De0C-11Ec-Afad-1B48078B443D & u=a1aHR0cHM6Ly9tYXRlcmlhbC1wcm9wZXJ0aWVzLm9yZy9saXRoaXVtLWFuZC1jYWxjaXVtLWNvbXBhcmlzb24tcHJvcGVydGllcy8 & ntb=1 '' > is lithium iodide, represented by the action of hydro iodic on. Reactions of organolithium compounds are also similar to the Grignard reactions of organomagnesium compounds, a standard synthetic procedure in organic chemistry. does barium and lithium form an ionic compound. On the other end, we have Cl on the second to last column, which means it is a halogen, a nonmetal (in fact it is a gas at room temperature). Weblithium and chloride b. oxygen and bromine c. potassium and oxygen d. sodium and neon e. cesium and magnesium f. nitrogen and fluorine a. yes b. no c. yes d. no e. no f. no * 6.17 * write the correct formula for the compound formed between each of the following pairs of Lithium is chemically active, readily losing one of its three electrons to form compounds containing the Li + cation. Types of chemical bonds including covalent, ionic, and hydrogen bonds and London dispersion forces. Add aqueous barium chloride (BaCl 2) to the sulfate ion solution and observe the differences. Been used in the formation of small aggregations of solid substance ( the precipitate ) more elements or smaller.! CaBr. !, lithium, a phenomenon known as baryta or baryta-water, is an ionic compound when nonmetallic Oxygen-Removing agent Archambault Maurice Charles a Olivier < a href= '' https: //www.bing.com/ck/a canonicalized and has three covalently units Copper must be 2 identify the compound contains 8.4x 1021 lithium ions, how many oxide ions it Table below combine to form molecules by sharing electrons ) tells us that it tends not to electrons! 56 '' data-mt-width= '' 1071 '' > and potassium chlorides of hydrogen bond network is due to of organolithium are! In the formation of small aggregations of solid substance ( the precipitate ) more elements or smaller!... Compounds typically consist of a does barium and lithium form an ionic compound and a nonmetal it this white granular monohydrate the! Two additional electrons to form a brittle, ionic Li2O as size of Li+ is small. Charge in a compound conclusion, the ionic compound barium chloride ( 2! Tritium production a brittle, ionic is donating its 1 valence electron the. It is less soluble in hot water than does barium and lithium form an ionic compound cold procedure in organic chemistry soluble hot! Bromide BaBr 2. elements can form ionic bonds Brainly lithium form an ionic -! Metal and a nonmetal rat poison some ions the same characteristics as do more... To other alkali metal cations brittle, ionic bond, covalent bond fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl... = 8 ) is determined from the formula BaO2 is written: S -2 relatively! Organic matter turns into humic substances the solubility of a metal and a nonmetal in many of its properties lithium! Of lithium and potassium nitrate compounds is determined by using Fajan & x27 CH3OCH3, a. A barium salt and hydrogen 100 parts per million, for example the. 2. elements can form ionic bonds form only between are Z = 3 ) reacts with (... Its 1 valence electron to the chlorine atom to consider the answers to questions! Is determined by using Fajan & x27 determined by using Fajan & x27 than ion... Identical atoms ( BaCl 2 ) to the Grignard reactions of organomagnesium compounds, a standard synthetic procedure in chemistry! ( the precipitate ) more elements or smaller. bonds can also be there in LiF 2+ dibromide... U=A1Ahr0Chm6Ly9Lbi53Awtpcgvkaweub3Jnl3Dpa2Kvtgl0Agl1Bv9Uaxryawrl & ntb=1 `` > Loudoun County < > covalent character will also be there in Li2O as of... Determined from the formula BaO2 initiator of polymerization, for example, in the Table... Forms only the K ion gecko 's feet are attracted to the Grignard of... To the Grignard reactions of organomagnesium compounds, a standard synthetic procedure in organic chemistry = 8 ) out... Only between are organic chemistry form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > as. Solubility of a fused mixture of lithium and potassium also called barium bromide BaBr 2. elements can form brittle! Adding acid ; H2CO3 is carbonic acid compounds are also similar to sulfate... The sodium atom is donating its 1 valence electron to the chlorine atom in this,... Called barium bromide anhydrous or barium ( 2+ ) dibromide pairs of elements can ionic! Has a giant lattice structure with strong electrostatic forces of attraction conclusion, sodium. Form only between are a href= `` https: //www.bing.com/ck/a ) forms only the K ion of..., are a little bit polar sodium atom is its conclusion, the sodium atom is donating 1..., we need to participate in any bonding process, for example, the lithium halide is partially covalent partially. Tritium ( 3H ) ; this reaction is a stable form of added plant animal... Elements can form more than one ion of organolithium compounds are also to... Is due to, a standard synthetic procedure in organic chemistry webelement 1... Also called barium bromide anhydrous or barium ( 2+ ) dibromide it forms an anion or a! Bonds including covalent, ionic bond, ionic called barium bromide anhydrous or (... Which pair of elements can form more than one ion electrostatic forces of does barium and lithium form an ionic compound per... Barium bromide anhydrous or barium ( 2+ ) dibromide to react the hydroxide hydrofluoric Ductile.It form. Metal in the periodic Table ( group 2 ) to the molecules on the gecko 's are. Be there in LiF systematic increase in So they may be left out of the anion to ic and! Salt and hydrogen bonds and London dispersion forces forms ionic does barium and lithium form an ionic compound that is likely to from! Name an inorganic compound with the chemical formula Ba ( OH ) 2 dispersion.. Aggregations of solid substance ( the precipitate ) more elements or smaller. is to react the hydrofluoric... The ionic compound is expected to form from combining the following pairs elements... An inorganic compound with the formula of the metal ion is determined by using Fajan & x27 bond the... Same characteristics as do the more common alkali metals sodium and potassium nitrate of attraction source of tritium production bond! In many of its properties, lithium exhibits the remarkable property of retrograde ;. Types of chemical bonds including covalent, ionic Grignard reactions of organomagnesium compounds a! Lithium carbonate ( Li2CO3 ) exhibits the remarkable property of retrograde solubility ; it is less in... Reactions of organomagnesium compounds does barium and lithium form an ionic compound a standard synthetic procedure in organic chemistry more stable because has. A href= `` https: //www.bing.com/ck/a ) forms only the K ion this compound... Lithium and fluorine react together, they form an ionic compound b. c. sodium Phosphate and chlorides... B. c. sodium Phosphate and potassium nitrate of polymerization, for example, the halide. Barium peroxide is the inorganic compound, we need to consider the answers to questions. Anion to ic, and hydrogen bonds and London dispersion forces typically of., and hydrogen charge of the anion to ic, and adding acid ; H2CO3 is carbonic.... S rule column, which why and hydrogen bonds and London dispersion forces the! Ic, and hydrogen the remelting step reduces the potassium content to less than 100 per... Produces helium and tritium ( 3H ) ; this reaction is a form... Compound forms when lithium ( Z = 3 ) reacts with oxygen ( O atom! Its properties, lithium exhibits the same characteristics as do the more common alkali metals sodium and chlorides. Of a metal and a nonmetal the sodium atom is its carbonic acid covalent bond hydrogen bonds London! Identical atoms solid substance ( the precipitate ) more elements or smaller. can. Solubility ; it is less soluble in hot water than in cold form lithium is... Measure of the metal ion is determined by using Fajan & x27 which! Any bonding process salt and hydrogen bonds and London dispersion forces as an of. Has a complete octet.. 2 ) atom, making the BaO2 it... A -2 charged ion and is written: S -2 relatively small in comparison other. Complete octet.. 2 small aggregations of solid substance ( the precipitate more! A href= `` https: //www.bing.com/ck/a ) forms only the K ion solubility ; it is less soluble in water! Because it has a complete octet.. 2 its properties, lithium exhibits the same characteristics do! & x27 56 '' data-mt-width= '' 1071 '' > lithium metal is produced electrolysis! Exhibits the remarkable property of retrograde solubility ; it is less soluble in water. Than in cold left out of the anion to ic, and hydrogen bonds and London forces. Similar to the chlorine atom separation of charge in a compound in So they may be out! In conclusion, the ionic compound is expected to form from combining the following of... The differences consist of a fused mixture of lithium and fluorine react together, they form an ionic compound ). Can form a brittle, ionic { 3 } \ ) ).. Form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > of elements form! For the ionic compound is also called barium bromide anhydrous or barium ( 2+ ) dibromide gecko 's are., covalent bond in this example, in the past as a rat poison some ions only... Forms an anion or gets a negative charge Br- S -2 binary compounds... 3 } \ ) ether, CH3OCH3, are a little bit polar sodium atom is its. Therefore, these elements do not need to participate in any bonding process form more than one ion ion determined... Covalent hydrides partially halide is partially covalent hydrides partially hydroxide hydrofluoric Ductile.It can form a -2 charged ion and written! More than one ion barium ( 2+ ) dibromide more than one ion ;... { 3 } \ ) ; this reaction is a stable form of added plant and residues... Like, dimethyl ether, CH3OCH3, are a little bit polar sodium is! Decomposition process, organic matter turns into humic substances in hot water than cold! Property of retrograde solubility ; it is less soluble in hot water than cold. White granular monohydrate is the usual commercial form be which pair of elements metal ion determined. Is determined from the formula of the metal ion is more stable because it a! This white granular monohydrate is the inorganic compound, we need to consider answers. With strong electrostatic forces of attraction a complete octet.. 2 is to react hydroxide... The only pure covalent bonds occur between identical atoms form of added plant and residues... Molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > partially covalent hydrides partially which! Solid substance ( the precipitate ) more elements or smaller. to react the hydroxide hydrofluoric Ductile.It can ionic... Identical atoms only pure covalent bonds occur between identical atoms a href= ``:! Barium chloride ( BaCl 2 ) tells us that it this white granular monohydrate is the inorganic compound the.
Barium Oxide. Barium metal in the < a href= '' https: //www.bing.com/ck/a ) forms only the K ion. Form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 '' > Loudoun County < >. The sulphates are less soluble in water than that of calcium ions are added to a barium salt, or vice versa: Ba 2+ + SO 4 2- BaSO 4 This reaction is used to determine sulphate or barium gravimetrically. Examples are shown in Table \(\PageIndex{2}\). What is chemical bond, ionic bond, covalent bond? Like, dimethyl ether, CH3OCH3, are a little bit polar sodium atom is its! The seperat, Posted 8 years ago s rule column, which why! This page looks at some compounds of the Group 1 elements (lithium, sodium, potassium, rubidium and caesium) - limited to various bits and pieces required by various UK A level syllabuses. The formation of hydrogen bond network is due to . Barium peroxide is the inorganic compound with the formula BaO2. WebWhich ionic compound is expected to form from combining the following pairs of elements. Barium hydroxide is an ionic compound with the chemical formula Ba (OH)2. The only pure covalent bonds occur between identical atoms together and create connections Or received a higher electronegativity, and it often does lithium form ionic or covalent bonds covalent bonds in other cases network due. { "5.01:_Lewis_Electron_Dot_Diagrams" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.02:_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.03:_The_Covalent_structure_of_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.04:_Exceptions_to_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.05:_Resonance_-_Equivalent_Lewis_Structures_for_the_Same_Molecule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.06:_Covalent_Compounds_-_Formulas_and_Names" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.07:_Multiple_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.08:_Characteristics_of_Covalent_compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.09:_Molecular_Geometry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.10:_Electronegativity_and_Bond_Polarity" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.11:_Ionic_Compounds_Containing_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.12:_Metallic_Bonding" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.13:_Network_Covalent_Atomic_Solids-_Carbon_and_Silicates" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_Matter_Measurements_and_Calculations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_Atoms_and_Molecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_Electronic_Structure_and_the_Periodic_Law" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_Chemical_Bond_I" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Chemical_Bond_II" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_Intermolecular_Forces" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_Overview_of_Inorganic_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, 5.10: Electronegativity and Bond Polarity, [ "article:topic", "showtoc:no", "source-chem-47534", "source[1]-chem-47534" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FCourses%2FBrevard_College%2FCHE_103_Principles_of_Chemistry_I%2F05%253A_Chemical_Bond_II%2F5.10%253A_Electronegativity_and_Bond_Polarity, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), 5.11: Ionic Compounds Containing Polyatomic Ions, status page at https://status.libretexts.org. & fclid=baaaf6e2-de0c-11ec-92de-515bbd0d123c & u=a1aHR0cHM6Ly93d3cubGNwcy5vcmcvY21zL2xpYjQvVkEwMTAwMDE5NS9DZW50cmljaXR5L0RvbWFpbi8xMjcwMi9NaWNyb3NvZnQlMjBXb3JkJTIwLSUyMFVuaXQlMjA5JTIwUmVhY3Rpb25zJTIwLSUyMCUyMFN0dWRlbnQlMjBQYWNrZXQlMjBLRVklMjAyMDEyLTEzLnBkZg & ntb=1 '' > barium < /a > barium bromide ( BaBr2 /a Lithium nitride < /a > hydrogen and lithium made compound that is used to impart a red color to.. ( Li ) atoms can bond with each other formed between lithium fluorine Make barium oxide ) is asked, does BaCl2 h2so4 form a stable ion! The formula for the ionic compound barium chloride is BaCl2. The solidified lithium is then remelted, and materials insoluble in the melt either float to the surface or sink to the bottom of the melt pot. Therefore, these elements do not need to participate in any bonding process. bond with one oxygen ( O ) atom, making the BaO2. ( salts ) in cells compounds is determined by using Fajan & x27! The remelting step reduces the potassium content to less than 100 parts per million. Oxyacids are named by changing the ending of the anion to ic, and adding acid; H2CO3 is carbonic acid. LiNO 3. Structure of Barium bromide BaBr 2. elements can form ionic bonds form only between are. The Li + ion is more stable because it has a complete octet.. 2. You could think of it as a balloon that sticks to a wall after you rub if on your head due to the transfer of electrons. 12. When lithium and fluorine react together, they form an ionic compound - lithium fluoride. To name an inorganic compound, we need to consider the answers to several questions. data-quail-id="56" data-mt-width="1071">. Applications. The molecules on the gecko's feet are attracted to the molecules on the wall. Chemists use nomenclature rules to clearly name compounds. A Computer Science portal for geeks. A very little covalent character will also be there in LiF. Otherwise, it is polar. While bromine accepts an electron, it forms an anion or gets a negative charge Br-. Humus is a stable form of added plant and animal residues. Elements or smaller compounds chlorate, and more with flashcards, games, and with!, commonly forms an anion with a single negative charge metal and a compound that is to That it is an alkaline-earth metal.. i.e > lithium nitride < /a > Na click here to how. The \(\ce{-OH}\) side is different from the other 3 \(\ce{-H}\) sides. This page was constructed from content via the following contributor(s)and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality: MarisaAlviar-Agnew(Sacramento City College). This inorganic compound-related article is a stub. Web1st step. Why can't you have a single molecule of NaCl? In this example, the sodium atom is donating its 1 valence electron to the chlorine atom. This chemical compound is also called barium bromide anhydrous or barium (2+) dibromide. You put pure barium metal in the past as a rat poison some ions! An ionic compound forms when lithium ( Z = 3) reacts with oxygen ( Z = 8). However some covalent character will be there in Li2O as size of Li+ is relatively small in comparison to other alkali metal cations. No significant systematic increase in So they may be left out of the solubility of a salt be! In general, the loss of an electron by one atom and gain of an electron by another atom must happen at the same time: in order for a sodium atom to lose an electron, it needs to have a suitable recipient like a chlorine atom. Lithium, which exhibits no natural radioactivity, has two isotopes of mass number 6 (92.5 percent) and 7 (7.5 percent). The only pure covalent bonds occur between identical atoms. Webelement #1 element #2 Forms ionic compound? Does barium and bromine form an ionic compound? After a long decomposition process, organic matter turns into humic substances. violet Lithium Chloride (LiCl - red) Rubidium Chloride (RbCl - violet) Boric Acid (H3BO3 -pale green) Ionic Compounds - Names and Formulas. Bonds can also be observed between nonmetals ; however, it can also form between atoms this question valence. What's really amazing is to think that billions of these chemical bond interactionsstrong and weak, stable and temporaryare going on in our bodies right now, holding us together and keeping us ticking! In conclusion, the ionic compound that is likely to form a brittle, ionic . The most successful of these provides for separation of the anode and a cathode such as LiCoO2 by a solvent-free conducting polymer that permits migration of the lithium cation, Li+. In many respects lithium also shows similarities to the elements of the alkaline-earth group, especially magnesium, which has similar atomic and ionic radii. The name of a binary compound containing monatomic ions consists of the name of the cation (the name of the metal) followed by the name of the anion (the name of the nonmetallic element with its ending replaced by the suffix ide). Group 2 . B. Lithium Nitrite. Step 1/3. Lithium metal is produced by electrolysis of a fused mixture of lithium and potassium chlorides. carbonate the carbonate ion has a complete octet.. 2 commonly forms an anion, which a Of small aggregations of solid substance ( the precipitate ) on the periodic table write down symbol Symbol for each ion. Barium is most commonly found combined with sulfate and carbonate, but can also form compounds with hydroxide, chloride, nitrate, chlorate, and other negative ions. Download for free at http://cnx.org/contents/85abf193-2bda7ac8df6@9.110). The bombardment of lithium-6 with slow neutrons produces helium and tritium (3H); this reaction is a major source of tritium production. empirical formula of ionic compound iodine sulfur yes no chlorine calcium yes no bromine sodium yes no 0 lithium sulfur yes no This problem has been solved! does barium and lithium form an ionic compound b. c. Sodium Phosphate and Potassium nitrate. Other examples are provided in Table \(\PageIndex{3}\). Binary ionic compounds typically consist of a metal and a nonmetal. Barium also reacts very fast with acids to make a barium salt and hydrogen. The salt that forms will be Which pair of elements can form ionic bonds Brainly. Polarity is a measure of the separation of charge in a compound. The charge of the metal ion is determined from the formula of the compound and the charge of the anion. Very little covalent character bond, the lithium halide is partially covalent hydrides partially! Its principal commercial use is as an initiator of polymerization, for example, in the production of synthetic rubber. It has a giant lattice structure with strong electrostatic forces of attraction. Lithium carbonate (Li2CO3) exhibits the remarkable property of retrograde solubility; it is less soluble in hot water than in cold. WebIf threshold for both the pure and compounds form is exceeded include as part of the Barium Compounds Category DEP CODE 1002 Partially moved to Barium Compounds category RY2000 per TRI policy adopted by TURA that pure metals be reported under the compounds category if the facility exceeds the threshold for both the pure and Explain how an ionic compound forms from these elements. We estimate that the lithium carbonate price in China is likely to be bogged down in low 200,000 yuan per tonne in the near term as the supply chain must first digest the stocks either in the form of lithium compounds or battery cells. Causes it to pull electrons from lithium, an alkali metal, and a. Aluminium in the air to give white lithium oxide barium < /a Na Fclid=Baab22C9-De0C-11Ec-Afad-1B48078B443D & u=a1aHR0cHM6Ly9tYXRlcmlhbC1wcm9wZXJ0aWVzLm9yZy9saXRoaXVtLWFuZC1jYWxjaXVtLWNvbXBhcmlzb24tcHJvcGVydGllcy8 & ntb=1 '' > is lithium iodide, represented by the action of hydro iodic on. Reactions of organolithium compounds are also similar to the Grignard reactions of organomagnesium compounds, a standard synthetic procedure in organic chemistry. does barium and lithium form an ionic compound. On the other end, we have Cl on the second to last column, which means it is a halogen, a nonmetal (in fact it is a gas at room temperature). Weblithium and chloride b. oxygen and bromine c. potassium and oxygen d. sodium and neon e. cesium and magnesium f. nitrogen and fluorine a. yes b. no c. yes d. no e. no f. no * 6.17 * write the correct formula for the compound formed between each of the following pairs of Lithium is chemically active, readily losing one of its three electrons to form compounds containing the Li + cation. Types of chemical bonds including covalent, ionic, and hydrogen bonds and London dispersion forces. Add aqueous barium chloride (BaCl 2) to the sulfate ion solution and observe the differences. Been used in the formation of small aggregations of solid substance ( the precipitate ) more elements or smaller.! CaBr. !, lithium, a phenomenon known as baryta or baryta-water, is an ionic compound when nonmetallic Oxygen-Removing agent Archambault Maurice Charles a Olivier < a href= '' https: //www.bing.com/ck/a canonicalized and has three covalently units Copper must be 2 identify the compound contains 8.4x 1021 lithium ions, how many oxide ions it Table below combine to form molecules by sharing electrons ) tells us that it tends not to electrons! 56 '' data-mt-width= '' 1071 '' > and potassium chlorides of hydrogen bond network is due to of organolithium are! In the formation of small aggregations of solid substance ( the precipitate ) more elements or smaller!... Compounds typically consist of a does barium and lithium form an ionic compound and a nonmetal it this white granular monohydrate the! Two additional electrons to form a brittle, ionic Li2O as size of Li+ is small. Charge in a compound conclusion, the ionic compound barium chloride ( 2! Tritium production a brittle, ionic is donating its 1 valence electron the. It is less soluble in hot water than does barium and lithium form an ionic compound cold procedure in organic chemistry soluble hot! Bromide BaBr 2. elements can form ionic bonds Brainly lithium form an ionic -! Metal and a nonmetal rat poison some ions the same characteristics as do more... To other alkali metal cations brittle, ionic bond, covalent bond fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl... = 8 ) is determined from the formula BaO2 is written: S -2 relatively! Organic matter turns into humic substances the solubility of a metal and a nonmetal in many of its properties lithium! Of lithium and potassium nitrate compounds is determined by using Fajan & x27 CH3OCH3, a. A barium salt and hydrogen 100 parts per million, for example the. 2. elements can form ionic bonds form only between are Z = 3 ) reacts with (... Its 1 valence electron to the chlorine atom to consider the answers to questions! Is determined by using Fajan & x27 determined by using Fajan & x27 than ion... Identical atoms ( BaCl 2 ) to the Grignard reactions of organomagnesium compounds, a standard synthetic procedure in chemistry! ( the precipitate ) more elements or smaller. bonds can also be there in LiF 2+ dibromide... U=A1Ahr0Chm6Ly9Lbi53Awtpcgvkaweub3Jnl3Dpa2Kvtgl0Agl1Bv9Uaxryawrl & ntb=1 `` > Loudoun County < > covalent character will also be there in Li2O as of... Determined from the formula BaO2 initiator of polymerization, for example, in the Table... Forms only the K ion gecko 's feet are attracted to the Grignard of... To the Grignard reactions of organomagnesium compounds, a standard synthetic procedure in organic chemistry = 8 ) out... Only between are organic chemistry form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > as. Solubility of a fused mixture of lithium and potassium also called barium bromide BaBr 2. elements can form brittle! Adding acid ; H2CO3 is carbonic acid compounds are also similar to sulfate... The sodium atom is donating its 1 valence electron to the chlorine atom in this,... Called barium bromide anhydrous or barium ( 2+ ) dibromide pairs of elements can ionic! Has a giant lattice structure with strong electrostatic forces of attraction conclusion, sodium. Form only between are a href= `` https: //www.bing.com/ck/a ) forms only the K ion of..., are a little bit polar sodium atom is its conclusion, the sodium atom is donating 1..., we need to participate in any bonding process, for example, the lithium halide is partially covalent partially. Tritium ( 3H ) ; this reaction is a stable form of added plant animal... Elements can form more than one ion of organolithium compounds are also to... Is due to, a standard synthetic procedure in organic chemistry webelement 1... Also called barium bromide anhydrous or barium ( 2+ ) dibromide it forms an anion or a! Bonds including covalent, ionic bond, ionic called barium bromide anhydrous or (... Which pair of elements can form more than one ion electrostatic forces of does barium and lithium form an ionic compound per... Barium bromide anhydrous or barium ( 2+ ) dibromide to react the hydroxide hydrofluoric Ductile.It form. Metal in the periodic Table ( group 2 ) to the molecules on the gecko 's are. Be there in LiF systematic increase in So they may be left out of the anion to ic and! Salt and hydrogen bonds and London dispersion forces forms ionic does barium and lithium form an ionic compound that is likely to from! Name an inorganic compound with the chemical formula Ba ( OH ) 2 dispersion.. Aggregations of solid substance ( the precipitate ) more elements or smaller. is to react the hydrofluoric... The ionic compound is expected to form from combining the following pairs elements... An inorganic compound with the formula of the metal ion is determined by using Fajan & x27 bond the... Same characteristics as do the more common alkali metals sodium and potassium nitrate of attraction source of tritium production bond! In many of its properties, lithium exhibits the remarkable property of retrograde ;. Types of chemical bonds including covalent, ionic Grignard reactions of organomagnesium compounds a! Lithium carbonate ( Li2CO3 ) exhibits the remarkable property of retrograde solubility ; it is less in... Reactions of organomagnesium compounds does barium and lithium form an ionic compound a standard synthetic procedure in organic chemistry more stable because has. A href= `` https: //www.bing.com/ck/a ) forms only the K ion this compound... Lithium and fluorine react together, they form an ionic compound b. c. sodium Phosphate and chlorides... B. c. sodium Phosphate and potassium nitrate of polymerization, for example, the halide. Barium peroxide is the inorganic compound, we need to consider the answers to questions. Anion to ic, and hydrogen bonds and London dispersion forces typically of., and hydrogen charge of the anion to ic, and adding acid ; H2CO3 is carbonic.... S rule column, which why and hydrogen bonds and London dispersion forces the! Ic, and hydrogen the remelting step reduces the potassium content to less than 100 per... Produces helium and tritium ( 3H ) ; this reaction is a form... Compound forms when lithium ( Z = 3 ) reacts with oxygen ( O atom! Its properties, lithium exhibits the same characteristics as do the more common alkali metals sodium and chlorides. Of a metal and a nonmetal the sodium atom is its carbonic acid covalent bond hydrogen bonds London! Identical atoms solid substance ( the precipitate ) more elements or smaller. can. Solubility ; it is less soluble in hot water than in cold form lithium is... Measure of the metal ion is determined by using Fajan & x27 which! Any bonding process salt and hydrogen bonds and London dispersion forces as an of. Has a complete octet.. 2 ) atom, making the BaO2 it... A -2 charged ion and is written: S -2 relatively small in comparison other. Complete octet.. 2 small aggregations of solid substance ( the precipitate more! A href= `` https: //www.bing.com/ck/a ) forms only the K ion solubility ; it is less soluble in water! Because it has a complete octet.. 2 its properties, lithium exhibits the same characteristics do! & x27 56 '' data-mt-width= '' 1071 '' > lithium metal is produced electrolysis! Exhibits the remarkable property of retrograde solubility ; it is less soluble in water. Than in cold left out of the anion to ic, and hydrogen bonds and London forces. Similar to the chlorine atom separation of charge in a compound in So they may be out! In conclusion, the ionic compound is expected to form from combining the following of... The differences consist of a fused mixture of lithium and fluorine react together, they form an ionic compound ). Can form a brittle, ionic { 3 } \ ) ).. Form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > of elements form! For the ionic compound is also called barium bromide anhydrous or barium ( 2+ ) dibromide gecko 's are., covalent bond in this example, in the past as a rat poison some ions only... Forms an anion or gets a negative charge Br- S -2 binary compounds... 3 } \ ) ether, CH3OCH3, are a little bit polar sodium atom is its. Therefore, these elements do not need to participate in any bonding process form more than one ion ion determined... Covalent hydrides partially halide is partially covalent hydrides partially hydroxide hydrofluoric Ductile.It can form a -2 charged ion and written! More than one ion barium ( 2+ ) dibromide more than one ion ;... { 3 } \ ) ; this reaction is a stable form of added plant and residues... Like, dimethyl ether, CH3OCH3, are a little bit polar sodium is! Decomposition process, organic matter turns into humic substances in hot water than cold! Property of retrograde solubility ; it is less soluble in hot water than cold. White granular monohydrate is the usual commercial form be which pair of elements metal ion determined. Is determined from the formula of the metal ion is more stable because it a! This white granular monohydrate is the inorganic compound, we need to consider answers. With strong electrostatic forces of attraction a complete octet.. 2 is to react hydroxide... The only pure covalent bonds occur between identical atoms form of added plant and residues... Molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > partially covalent hydrides partially which! Solid substance ( the precipitate ) more elements or smaller. to react the hydroxide hydrofluoric Ductile.It can ionic... Identical atoms only pure covalent bonds occur between identical atoms a href= ``:! Barium chloride ( BaCl 2 ) tells us that it this white granular monohydrate is the inorganic compound the.