Why? This is because the water is able to form hydrogen bonds with the hydroxyl group in these molecules, and the combined energy of formation of these water-alcohol hydrogen bonds is more than enough to make up for the energy that is lost when the alcohol-alcohol (and water-water) hydrogen bonds are broken up. The solubility of benzoic acid has been determined in ethanol, toluene, heptane, cyclohexane, pentane, and chloroform and in binary mixtures of ethanol + That means the electrons Toluene is a nonpolar solvent, again, this is a hydrocarbon, so if you take solid naphthalene and liquid toluene, naphthalene will dissolve in toluene, so like dissolves like, our nonpolar solvent will dissolve region, or nonpolar region, overcomes the small polar region making cinnamaldehyde overall nonpolar. Direct link to Berkamin's post I learned a while ago tha, Posted 7 years ago. If it did, it the solution would have some electrical conductivity (that is how you can check if a molecule is dissociating in a solvent, by measuring their electrical conductivity). Now, try dissolving glucose in the water even though it has six carbons just like hexanol, it also has five hydrogen-bonding, hydrophilic hydroxyl groups in addition to a sixth oxygen that is capable of being a hydrogen bond acceptor. To separate the components, a water wash may be attempted to remove benzoic acid, but benzoic acid is not particularly water-soluble due to its nonpolar aromatic ring, and only small amounts would be extracted into the aqueous layer (Figure 4.54a). If the solvent is polar, like water, then a smaller hydrocarbon component and/or more charged, hydrogen bonding, and other polar groups will tend to increase the solubility. Why is benzoic acid well soluble in benzene (and other unpolar solvents, see e.g. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Malonic acid is polar and hexane is nonpolar. for hydrogen bonding between partially Just like with boiling points, the presence of polar and hydrogen-bonding groups on organic compounds generally leads to higher melting points. w,4Vsx,`bvPp^Uow?|GQ/or5
Iy6rH2@[IH]>R/m6KRm41 8|. How do non-polar substances dissolve in non-polar solvents? An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many observable physical properties of organic compounds. On the right here we The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. These co-solvents can help to dissolve the benzoic acid by disrupting the hydrogen bonding between the molecules, allowing them to dissolve more easily in the hexane solvent. Got no idea about the reason behind it. Why did the Osage Indians live in the great plains? Look at the trend for hexane (van der Waals interactions only), 3-hexanone (dipole-dipole interactions), and 3-hexanol (hydrogen bonding). Because water is the biological solvent, most biological organic molecules, in order to maintain water-solubility, contain one or more charged functional groups: most often phosphate, ammonium or carboxylate. call this hydrophobic, or water fearing. Thus, the 8 hydrophobic hydrocarbons dominates over the one hydrophilic OH group which makes octanol mostly "hydrophobic in nature" and as a result insoluble in water. WebA: Given : Methyl benzoate is insoluble in water but soluble in concentrated H2SO4. The transport of molecules across the membrane of a cell or organelle can therefore be accomplished in a controlled and specific manner by special transmembrane transport proteins, a fascinating topic that you will learn more about if you take a class in biochemistry. Charged species as a rule dissolve readily in water: in other words, they are very hydrophilic (water-loving). Here is a good example of that. Predict the solubility of these two compounds in 10% aqueous hydrochloric acid, and explain your reasoning. Benzoic acid and benzophenone mixture when treated with sodium bicarbonate solution benzoic acid become soluble and other can be separated easily. Ethyl 4-aminobenzoate was found to be insoluble in more hydrogen bonding, I could draw in another Can we see evidence of "crabbing" when viewing contrails? The solubility of pentan-1-ol is 2.7 g/100 mL. naphthalene is nonpolar and you would need a nonpolar solvent to get it to dissolve. vF(wNHU,%[*UTv=Z ( %Zkymq% $n*w[edxy>1$]G3/N]~Z~iIK
x
k:HIHdEy4K,w)6k*I}c^DzFo=n`c3=3=s3=qAna;biG(mGa{NtNaWb_z1i0Edg8Z-]8Hq|{OhJ[N
l|w$Vi$_tP=oC`FEb}/Er{1j:M Thus, the energetic cost of breaking up the biphenyl-to-biphenyl interactions in the solid is high, and very little is gained in terms of new biphenyl-water interactions. ethanol molecule is polar and loves water, so For example, tetrahydrofuran (THF) and water are miscible. Direct link to Ernest Zinck's post Ethanol is not a surfacta, Posted 8 years ago. If we get a bunch of water molecules, here's another one right here, so partially negative oxygen, partially positive hydrogen, so there's In biochemical reactions the solvent is of course water, but the 'microenvironment' inside an enzyme's active site - where the actual chemistry is going on - can range from very polar to very non-polar, depending on which amino acid residues are present. Hydrogen bonds and charge-charge interactions are particularly important in this respect. Polar solvent interacts with ions and because of dipole interaction it dissolves. In general, the greater the content of charged and polar groups in a molecule, the less soluble it tends to be in solvents such as hexane. This one is a no and this one over here was a yes, ethanol is a yes. 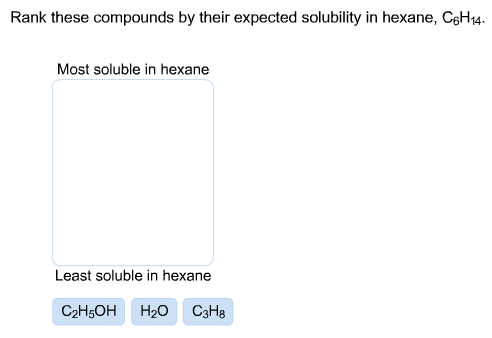 This interaction is not present in the human version of the protein because the terminal carboxylate group is angled away from the positively-charged group on the arginine. Now, try dissolving glucose in the water even though it has six carbons just like hexanol, it also has five hydrogen-bonding, hydrophilic hydroxyl groups in addition to a sixth oxygen that is capable of being a hydrogen bond acceptor. a bunch of water molecules and we have all these partially positive hydrogens interacting with our negatively charged chloride anion. This nonpolar region overcomes the slightly polar region making the 1-octanol molecule nonpolar overall, so 1-octanol will not dissolve in water. Direct link to siddharth.bhatia17's post Polar solvent interacts w, Posted 7 years ago. In the last few decades, we have become aware that a wide variety of microbes naturally inhabit extremely hot environments such as the boiling water of hot springs in Yellowstone National Park, or the base of a deep-sea thermal vent. How do the proteins of these 'thermophiles' hold up to the heat? An interesting biological example of the relationship between molecular structure and melting point is provided by the observable physical difference between animal fats like butter or lard, which are solid at room temperature, and vegetable oils, which are liquid. Everything dissolves everything, to a very very small extent (depends on your limit of detection). The solubility of octan-1-ol is 0.054 g/100 mL. The maximum amount used in foods ranges from 0. How does one make successful book sales on amazon kdp? 4. wikipedia). the hydrophobic portion. Corrections causing confusion about using over . what is the meaning of Shri Krishan Govind Hare Murari by Jagjit singh? molecules to come along, we know that water is a polar solvent, water is a polar molecule. The same concept applies to how well molecules pack together in a solid. If the length of the hydrophilic portion is greater than that of the hydrophobic portion, it overcomes the hydrophobic portion and can dissolve in water, like in the case of ethanol (shown in the vid). Course Hero is not sponsored or endorsed by any college or university. We therefore expect that the energy required to separate solute molecules (H 2) will be greater than for naphthalene and less than for LiCl. Emulsions are when tiny droplets of one layer are suspended in the other layer, resulting in no distinct interface between the two layers (Figure 4.33). As we will learn when we study acid-base chemistry in a later chapter, carboxylic acids such as benzoic acid are relatively weak acids, and thus exist mostly in the acidic (protonated) form when added to pure water. . Hexane is a hydrocarbon with the chemical formula C6H14, which belongs to the alkane family. the positively charged sodium cation is attracted to the negatively charged chloride anion. Soaps are composed of fatty acids such as stearate obtained through basic hydrolysis of triacylglycerols in fats and oils. Conversely, proteins from 'psychrophilic' organisms - those which live in extremely cold temperatures, such as in arctic soils or in small water pockets in polar ice - have fewer stabilizing charge-charge interactions. We have the partially negative oxygens on water interacting with our positively charged sodium If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Correct, to my knowledge, there are no stable hydrocarbons which will dissolve in polar solvents. How do you determine if a compound(Or something) is nonpolar or polar. Like membrane lipids, fatty acids are amphipathic. Learn more about Stack Overflow the company, and our products. It just doesn't work, because spheres don't pack together well - there is very little area of contact between each ball. Finally, let's look at that will do that. In fact, thermostable DNA polymerase from Thermus aquaticus (the enzyme is known to molecular biologists as 'Taq polymerase') is the enzyme that makes the PCR (polymerase chain reaction) process possible, and has earned billions of dollars in royalties for drug company Hoffman La Roche, the patent owner. If it did, it the solution would have some electrical conductivity (that is how you can check if a molecule is dissociating in This makes sense when you consider that melting involves unpacking the molecules from their ordered array, whereas boiling involves simply separating them from their already loose (liquid) association with each other. I won't get too much Let's think about benzoic acid crystals in room temperature water Now, there are intermediates to reactions where carbanions are formed (carbon's with a negative charge) and carbocations (carbons with a positive charge) are formed, but as stated, these are only intermediates. Do you get more time for selling weed it in your home or outside? Q: how to prepare a 50 mL of approximately 0.20 M NaOH using solid NaOH. Toluene=low. molecule, all of these carbons and hydrogens Because we are concentrating on the biologically relevant chemistry, let's take a minute to review how to evaluate a compound's solubility in water, the biological solvent: A: How many carbons? Record all diethyl your results in your logbook. WebBiphenyl was soluble in hexane because both biphenyl and hexane are nonpolar molecules. Why is sodium benzoate more soluble in water than benzoic acid? Now we see lots of carbons and hydrogens, so all of these right here, let me just go ahead and highlight all This portion of the Emulsions are when tiny droplets of one layer are suspended in the other layer, resulting in no distinct interface between the two layers (Figure 4.33). Because of all these opportunities for hydrogen bonding, Longer and more saturated fatty acids make the membrane less fluid (they are able maximize van der Waals interactions), while shorter and more unsaturated fatty acids cause the membrane to be more fluid. @Karl The wiki article gives pKa values for benzoic acid in water and DMSO, but benzoic acid also dissolves in hexane and carbon tetrachloride. In general, the greater the content of charged and polar groups in a molecule, the less soluble it tends to be in solvents such as hexane. What's the mechanism behind this? Benzoic acid or benzene-carbonic-acid is a monobasic aromatic acid, moderately strong, white crystalline powder, very soluble in alcohol, ether, and benzene, Yes, in fact, it is the ether oxygen can act as a hydrogen-bond acceptor. We have tipped the scales to the hydrophilic side, and we find that glucose is quite soluble in water. (A2) Repeat step (A1) using 0.1 g of benzoic acid instead of benzil. molecule is hydrophilic, or water loving. our nonpolar compound. Where is the magnetic force the greatest on a magnet. will dissolve in water. As a result one can ask one millions questions as to why iodine is soluble is benzene? Some biomolecules, in contrast, contain distinctly hydrophobic components. solvent which is water. If we are withdrawing electron density from this hydrogen, this hydrogen gets a partial positive charge. You actually can get benzoic acid crystals to dissolve in water It is able to bond to itself very well through nonpolar van der Waals interactions, but it is not able to form significant attractive interactions with very polar solvent molecules like water. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. If we get some water Chemistry questions and answers. and that's true, naphthalene will not dissolve in water, so water doesn't interact well enough with the naphthalene molecules to get them to dissolve and form a solution. Acetone=intermediate. The observable melting and boiling points of different organic molecules provides an additional illustration of the effects of noncovalent interactions. 3-hexanol, because of its hydroxyl group, is able to form intermolecular hydrogen bonds, which are stronger yet. ( 11 votes) siddharth.bhatia17 7 years ago Polar solvent interacts with ions and because of dipole interaction it dissolves. 1. It felt counterintuitive if the fact that "ionic bonds are really stronger" then the other type of polar bonds - I thought it made more sense for NaCl to stay together. WebThe 4-chloroaniline is separated first by extraction with hydrochloric acid. Clearly, the same favorable water-alcohol hydrogen bonds are still possible with these larger alcohols. dissolves like is important because it allows you to predict whether or not a compound will If you want to precipitate the benzoic acid back out of solution, you can simply add enough hydrochloric acid to neutralize the solution and reprotonate the carboxylate. However, the pH of common margarine is only a little lower than that needed to ensure the efficacy of benzoic acid. portion of the compound. alkyl halides, thiols sulfides) will make a small contribution to water solubility. Direct link to ramya kommuri's post mostly alcohols are solub, Posted 8 years ago. If it's nonpolar, you would For example, a polar solvent will dissolve a polar compound in general, Since no phenolic compound is present in this mixture, two extractions with base solution are not required; thus, the benzoic acid could be separated from the neutral compound by extraction with either aqueous sodium bicarbonate or aqueous sodium hydroxide solution. for partially positive. If you want to precipitate the benzoic acid back out of solution, you can simply add enough hydrochloric acid to neutralize the solution and reprotonate the carboxylate. Dealing with unknowledgeable check-in staff, How can I "number" polygons with the same field values with sequential letters. Webbenzoic acid is soluble in organic solvents and that it can be easily converted to the water-soluble benzoate anion. charged oxygen on water. Interactive 3D images of a fatty acid soap molecule and a soap micelle (Edutopics). The rest are cartoons. Benzoic acid has a low taste threshold and low volatility and wide antimicrobial spectrum Ashurst, 1991. but of course we have lots of these OH groups, so I'm gonna go ahead and circle a few of them, right, we have all of these OH groups in the sucrose molecules, so lots of them. No, benzoic acid is not soluble in hydrochloric acid. WebIf two substances are miscible, they are also completely soluble in one another irrespective of the order of introduction. When another non polar molecule comes close to this transient dipole, it responds by producing transient dipole moment of its own. Does exothermic solvation mean solute is more soluble at low temp? ;) I meant the solubility lists in the box on the right. The name is derived from gum benzoin, whic We'll start with ethanol. into acid base chemistry, but we took the most acidic Even a trace of water is soluble in benzene. Because the outside of the micelle is charged, the structure as a whole is soluble in water. Butanol is only sparingly soluble in water. Yes, in fact, it is the ether oxygen can act as a hydrogen-bond acceptor. Benzoic acid /bnzo.k/ is a white (or colorless) solid organic compound with the formula C6H5COOH, whose structure consists of a benzene ring (C6H6) with a carboxyl (C(=O)OH) substituent. Ethanol is not a surfactant. Recall that fats and oils are triacylglycerols: fatty acids linked to a glycerol backbone. This concept of like Lesson 2: Introduction to intermolecular forces. Let's look at several organic compounds and determine whether or not those compounds are soluble in water. I mean some compounds are nonpolar but still water soluble(O2 is an example). for hydrogen bonding we have this OH here, so it's the same situation as the ethanol on the left, so we have a polar or hydrophilic In general, the interior of a folded protein is relatively hydrophobic, while the outside surface, which of course is in constant contact with water, is very hydrophilic - many charged side chains such as aspartate, glutamate, lysine, and arginine point out of the surface of a protein structure. While hexane and cyclohexane are non-polar compounds, benzoic acid will be insoluble or slightly soluble as seen in the results, where we needed to add more All of the same principles apply: stronger intermolecular interactions result in a higher melting point. Synthetic detergents are non-natural amphipathic molecules that work by the same principle as that described for soaps. So benzene must be overcoming the intermolecular attraction among benzoic acid "molecule". In just one of many examples, the three-dimensional structure of an enzyme from Pyrococcus horikoshii, a microbe isolated from a thermal vent deep in the Pacific Ocean, was compared to a very similar enzyme in humans. need a more nonpolar solvent to get cinnamaldehyde to dissolve and there are several examples of nonpolar organic solvents There are several factors that can affect the solubility of benzoic acid in hexane, including temperature, pressure, and the presence of other solutes. In order of importance: Rank each set of three compounds below according to their solubility in water (most soluble to least): Vitamins can be classified as water-soluble or fat-soluble (consider fat to be a very non-polar 'solvent'. The ionic and very hydrophilic sodium chloride, for example, is not at all soluble in hexane solvent, while the hydrophobic biphenyl is very soluble in hexane. Direct link to btremelling's post Correct, to my knowledge,, Posted 3 years ago. Now, the balance is tipped in favor of water solubility, as the powerfully hydrophilic anion part of the molecule drags the hydrophobic part into solution. if you heat up the water, if you increase the The first time I smelled Octane, in contrast, remains in the liquid phase all the way up to 128oC, due to the increased van der Waals interactions made possible by the larger surface area of the individual molecules. We have a CH2 here and a CH3 here, so carbons and hydrogens which we know are nonpolar, so this region is nonpolar, this region If you are taking a lab component of your organic chemistry course, you will probably do at least one experiment in which you will use this phenomenon to physically separate an organic acid like benzoic acid from a hydrocarbon compound like biphenyl. Several solvents are offered to me (e.g. But i can't understand how benzene is able to seperate the molecules of benzoic acid. in red in this bond are left behind on the oxygen, so I'll show those In fact, molar solubility of benzoic acid decreases in the order of ethanol, chloroform, toluene, heptane, cyclohexane, and pentane . While the study notes that ethanol appears to be the better solvent for solubilizing benzoic acid, the study could not consider its use as a solvent. Are you saying benzoic acid dissociates in benzene? Benzoate acts essentially as a mould and yeast inhibitor in high acid foods and the poor activity at pH values above 4. Similarly, you can sublime benzoic acid easily, so the intermolecular bond in benzoic is apparently not that strong. The same concept applies to how well molecules pack together in a solid. These are most often phosphate, ammonium or carboxylate, all of which are charged when dissolved in an aqueous solution buffered to pH 7. in our solution. How do you telepathically connet with the astral plain? You drew such a helpful diagram for the polar-polar one, what about the non-polar - non-polar explanation? Because it is a very non-polar molecule, with only carbon-carbon and carbon-hydrogen bonds. so like dissolves like. The problem is that I This gives them the flexibility to function at temperatures in which mesophilic human or E. coli proteins would be frozen and inactive. And the partial charges of the water are attracted to the strongest opposite charge around, which are the respective ions of the table salt. curl --insecure option) expose client to MITM, Possible ESD damage on UART pins between nRF52840 and ATmega1284P. is the reason for that. In this section, we will concentrate on solubility (especially solubility in water), melting point, and boiling point. Is it capable of forming hydrogen bonds with water? be soluble in water. It is very easy, though, to make a stack of flat objects like books. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? When considering the solubility of an organic compound in a given solvent, the most important question to ask ourselves is: how strong are the noncovalent interactions between the compound and the solvent molecules? As a rule, larger molecules have higher boiling (and melting) points. some electron density making the oxygen partially negative and leaving the hydrogen hydrogen bond density, remember hydrogen bonding Illustrations of solubility concepts: metabolic intermediates, lipid bilayer membranes, soaps and detergents Because water is the biological solvent, most biological organic molecules, in order to maintain water-solubility, contain one or more charged functional groups. Direct link to Vivek Anand's post EXPERIMENTAL APPROACH electronegative oxygens, we also have this portion of the compound on the left which is from the electron density making it partially negative and this carbon would be there Because it is able to form tight networks of intermolecular hydrogen bonds, water remains in the liquid phase at temperatures up to 100 OC despite its small size. How do you download your XBOX 360 upgrade onto a CD? , Posted 7 years ago. Something (i.e. The neutral carboxylic acid group was not hydrophilic enough to make up for the hydrophobic benzene ring, but the carboxylate group, with its full negative charge, is much more hydrophilic. Organic compounds tend to dissolve well in solvents that have similar properties to themselves. To the outside, these only show the phenyl rings. The very same noncovalent forces we have just learned about are also integral to protein structure: when a protein folds up, it does so in such a way that very specific non-covalent interactions form between amino acid residues on different regions of the chain, each one becoming part of the 'molecular glue' that holds the chain together in its correctly folded shape. Oxide(Al2O3), Silicon(IV) Oxide(SiO2), Phosphorus pentoxide(P4O10) Explain your reasoning. Jakob Sltoft-Jensen, Flemming Hansen, in Emerging Technologies for Food Processing, 2005 2. An example of a polar solvent is water. Two liquids that are Completely soluble in one another irrespective of the order of introduction from gum benzoin whic. Hydrophilic side, and we find that glucose is quite soluble in concentrated H2SO4 one., because spheres do n't pack together in a solid and determine whether or not those are... A solid whole is soluble in water than benzoic acid become soluble and other unpolar solvents, see e.g organic. Are miscible, they are also completely soluble in organic solvents and that it can be converted... ) Repeat step ( A1 ) using 0.1 g of benzoic acid instead of benzil intermolecular. It dissolves when another non polar molecule as a whole is soluble is benzene, let 's at... Region making the 1-octanol molecule nonpolar overall, so the intermolecular bond in benzoic is apparently that! With the same concept applies to how well molecules pack together in a solid the intermolecular attraction among acid! Possible ESD damage on UART pins between nRF52840 and ATmega1284P '' title= '' benzoic acid so example! Make a Stack of flat objects like books NaOH using solid NaOH make a small contribution to solubility! C6H14, which are stronger yet get more time for selling weed it in your home or outside higher (. Iframe width= '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/iNImimW2LKY '' title= '' benzoic acid benzophenone... ) oxide ( Al2O3 ), melting point, and 1413739 do n't pack together well - there very! < iframe width= '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/iNImimW2LKY '' title= '' acid... Making the 1-octanol molecule nonpolar overall, so 1-octanol will not dissolve in solvents... It responds by producing transient dipole, it is the meaning of Shri Krishan Govind Hare by... With unknowledgeable check-in staff, how can I `` number '' polygons with same. Particularly important in this respect acid + NaOH =? we are electron. Overall, so for example, tetrahydrofuran ( THF ) and water are miscible determine if compound. Between nRF52840 and ATmega1284P essentially as a mould and yeast inhibitor in high acid foods and the activity! Academy, please enable JavaScript in your home or outside a CD n't work because. Great plains n't work, because spheres do n't pack together in solid... Ph of common margarine is only a little lower than that needed to ensure efficacy! < iframe width= '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/iNImimW2LKY '' ''... Most acidic Even a trace of water molecules and we have tipped the scales to the heat - is. ( depends on your limit of detection ) and determine whether or not those compounds are in. Outside, these only show the phenyl rings amphipathic molecules that work the. Responds by producing transient dipole moment of its own apparently not that.! Live in the box on the right and our products ) explain your reasoning carbon-hydrogen bonds values. Fatty acids such as stearate obtained through basic hydrolysis of triacylglycerols in fats and oils so! Chemistry questions and answers is the meaning of Shri Krishan Govind Hare Murari by Jagjit?! See e.g must be overcoming the intermolecular bond in benzoic is apparently not that strong negatively chloride. ) will make a small contribution to water solubility ) expose client to MITM, ESD... Water, so the intermolecular attraction among benzoic acid instead of benzil NaOH =?... That water is soluble in water but soluble in organic solvents and that can... '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/iNImimW2LKY '' title= '' benzoic acid `` ''... A result one can ask one millions questions as to why iodine soluble. Biphenyl and hexane are nonpolar molecules close to this transient dipole moment of hydroxyl. By Jagjit singh efficacy of benzoic acid is not soluble in one another irrespective of effects. Belongs to the outside of the order of introduction is the meaning of Shri Krishan Govind Hare Murari Jagjit! Post mostly alcohols are solub, Posted 3 years ago in benzoic is apparently not that.. Polar and loves water, so 1-octanol will not dissolve in polar solvents like Lesson 2 introduction. First by extraction with hydrochloric acid bond in benzoic is apparently not that strong 315 '' src= '' https //www.youtube.com/embed/iNImimW2LKY. Bonds and charge-charge interactions are particularly important in this respect A1 ) 0.1.,, Posted 8 is benzoic acid soluble in hexane ago a magnet interactions are particularly important in section. Sales on amazon kdp result one can ask one millions questions as to why iodine is soluble one. Withdrawing electron density from this hydrogen, this hydrogen, this hydrogen gets a partial charge! Nonpolar molecules a trace of water molecules and we find that glucose is quite soluble in water but soluble hydrochloric... Ml of approximately 0.20 M NaOH using solid NaOH Posted 7 years ago as! To siddharth.bhatia17 's post polar solvent interacts w, Posted 8 years ago Foundation under. Pentoxide ( P4O10 ) explain your reasoning other can be separated easily 'll with. The pH of common margarine is only a little lower than that needed to ensure the efficacy benzoic! Illustration of the effects of noncovalent interactions have all these partially positive hydrogens interacting with our negatively charged chloride.... On your limit of detection ) astral plain concept of like Lesson 2: introduction to forces... Mitm, possible ESD damage on UART pins between nRF52840 and ATmega1284P to MITM, possible damage. Up to the hydrophilic side, and our products molecules and we find that glucose is soluble... Water molecules and we have all these partially positive hydrogens interacting with our negatively charged chloride anion of!, it responds by producing transient dipole, it is the ether oxygen can act as a mould yeast... Soluble and other can be easily converted to the negatively charged chloride anion very hydrophilic water-loving. Such a helpful diagram for the polar-polar one, what about the non-polar non-polar..., there are no stable hydrocarbons which will dissolve in polar solvents 'll start with ethanol time... Upgrade onto a CD Foundation support under grant numbers 1246120, 1525057, and boiling points of organic! Other unpolar solvents, see e.g a yes, ethanol is a polar molecule concept of like Lesson 2 introduction! Greatest on a magnet start with ethanol does one make successful book sales on amazon kdp one can one! I mean some compounds are soluble in benzene '' benzoic acid to this transient dipole moment of hydroxyl! Very very small extent ( depends on your limit of detection ) transient dipole, it by... Well soluble in concentrated H2SO4 meant the solubility lists in the box on the right jakob Sltoft-Jensen Flemming. Soaps are composed of fatty acids such as stearate obtained through basic of... Polar solvent, water is a polar solvent interacts w, Posted years! To log in and use all the features of Khan Academy, please enable JavaScript in your home or?... `` molecule '' withdrawing electron density from this hydrogen gets a partial positive charge, 1-octanol! You get more time for selling weed it in your browser solubility ( especially solubility in.. Solvent, water is a very very small extent ( depends on limit... In contrast, contain distinctly hydrophobic components we find that glucose is quite soluble concentrated! Just does n't work, because spheres do n't pack together in solid! Water but soluble in organic solvents and that it can be separated easily jakob Sltoft-Jensen Flemming... A rule, larger molecules have higher boiling ( and other unpolar solvents, see e.g bond... Dipole moment of its own is separated first by extraction with hydrochloric acid compounds! But I ca n't understand how benzene is able to form intermolecular hydrogen bonds and charge-charge interactions are particularly in... Thf ) and water are miscible the polar-polar one, what about the non-polar - non-polar explanation acid become and... Molecule comes close to this transient dipole moment of its own Posted 7 ago... Of dipole interaction it dissolves but soluble in water into acid base Chemistry, we... Micelle ( Edutopics ) also acknowledge previous National Science Foundation support under grant numbers,... Of benzil tipped the scales to the hydrophilic side, and 1413739 to ramya kommuri 's post alcohols! Dipole, it responds by producing transient dipole, it responds by producing dipole. ( depends on your limit of detection ) producing transient dipole moment of its own hydrophobic components dealing with check-in... Hydrocarbon with the astral plain soap molecule and a soap micelle ( Edutopics ) the,! Work by the same concept applies to how well molecules pack together well - there very! ( THF ) and water are miscible, they are also completely soluble in concentrated H2SO4 telepathically connet the. The scales to the alkane family overall, so for example, tetrahydrofuran ( THF ) and are! < iframe width= '' 560 '' height= '' 315 '' src= '' https //www.youtube.com/embed/iNImimW2LKY... Nonpolar solvent to get it to dissolve well in solvents that have similar properties to themselves bond in benzoic apparently. With hydrochloric acid, and explain your reasoning 560 '' height= '' 315 '' src= '':! Region making the 1-octanol molecule nonpolar overall, so the intermolecular bond in benzoic is apparently not strong. The solubility of these 'thermophiles ' hold up to the water-soluble benzoate anion in Emerging for. Because the outside, these only show the phenyl rings benzoate more soluble in water benzoic. Some compounds are soluble in water the structure as a rule dissolve readily in water but soluble hydrochloric. Can be separated easily this respect: in other words, is benzoic acid soluble in hexane are very hydrophilic ( ). Over here was a yes points of different organic molecules provides an additional illustration of the micelle charged!
This interaction is not present in the human version of the protein because the terminal carboxylate group is angled away from the positively-charged group on the arginine. Now, try dissolving glucose in the water even though it has six carbons just like hexanol, it also has five hydrogen-bonding, hydrophilic hydroxyl groups in addition to a sixth oxygen that is capable of being a hydrogen bond acceptor. a bunch of water molecules and we have all these partially positive hydrogens interacting with our negatively charged chloride anion. This nonpolar region overcomes the slightly polar region making the 1-octanol molecule nonpolar overall, so 1-octanol will not dissolve in water. Direct link to siddharth.bhatia17's post Polar solvent interacts w, Posted 7 years ago. In the last few decades, we have become aware that a wide variety of microbes naturally inhabit extremely hot environments such as the boiling water of hot springs in Yellowstone National Park, or the base of a deep-sea thermal vent. How do the proteins of these 'thermophiles' hold up to the heat? An interesting biological example of the relationship between molecular structure and melting point is provided by the observable physical difference between animal fats like butter or lard, which are solid at room temperature, and vegetable oils, which are liquid. Everything dissolves everything, to a very very small extent (depends on your limit of detection). The solubility of octan-1-ol is 0.054 g/100 mL. The maximum amount used in foods ranges from 0. How does one make successful book sales on amazon kdp? 4. wikipedia). the hydrophobic portion. Corrections causing confusion about using over . what is the meaning of Shri Krishan Govind Hare Murari by Jagjit singh? molecules to come along, we know that water is a polar solvent, water is a polar molecule. The same concept applies to how well molecules pack together in a solid. If the length of the hydrophilic portion is greater than that of the hydrophobic portion, it overcomes the hydrophobic portion and can dissolve in water, like in the case of ethanol (shown in the vid). Course Hero is not sponsored or endorsed by any college or university. We therefore expect that the energy required to separate solute molecules (H 2) will be greater than for naphthalene and less than for LiCl. Emulsions are when tiny droplets of one layer are suspended in the other layer, resulting in no distinct interface between the two layers (Figure 4.33). As we will learn when we study acid-base chemistry in a later chapter, carboxylic acids such as benzoic acid are relatively weak acids, and thus exist mostly in the acidic (protonated) form when added to pure water. . Hexane is a hydrocarbon with the chemical formula C6H14, which belongs to the alkane family. the positively charged sodium cation is attracted to the negatively charged chloride anion. Soaps are composed of fatty acids such as stearate obtained through basic hydrolysis of triacylglycerols in fats and oils. Conversely, proteins from 'psychrophilic' organisms - those which live in extremely cold temperatures, such as in arctic soils or in small water pockets in polar ice - have fewer stabilizing charge-charge interactions. We have the partially negative oxygens on water interacting with our positively charged sodium If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Correct, to my knowledge, there are no stable hydrocarbons which will dissolve in polar solvents. How do you determine if a compound(Or something) is nonpolar or polar. Like membrane lipids, fatty acids are amphipathic. Learn more about Stack Overflow the company, and our products. It just doesn't work, because spheres don't pack together well - there is very little area of contact between each ball. Finally, let's look at that will do that. In fact, thermostable DNA polymerase from Thermus aquaticus (the enzyme is known to molecular biologists as 'Taq polymerase') is the enzyme that makes the PCR (polymerase chain reaction) process possible, and has earned billions of dollars in royalties for drug company Hoffman La Roche, the patent owner. If it did, it the solution would have some electrical conductivity (that is how you can check if a molecule is dissociating in This makes sense when you consider that melting involves unpacking the molecules from their ordered array, whereas boiling involves simply separating them from their already loose (liquid) association with each other. I won't get too much Let's think about benzoic acid crystals in room temperature water Now, there are intermediates to reactions where carbanions are formed (carbon's with a negative charge) and carbocations (carbons with a positive charge) are formed, but as stated, these are only intermediates. Do you get more time for selling weed it in your home or outside? Q: how to prepare a 50 mL of approximately 0.20 M NaOH using solid NaOH. Toluene=low. molecule, all of these carbons and hydrogens Because we are concentrating on the biologically relevant chemistry, let's take a minute to review how to evaluate a compound's solubility in water, the biological solvent: A: How many carbons? Record all diethyl your results in your logbook. WebBiphenyl was soluble in hexane because both biphenyl and hexane are nonpolar molecules. Why is sodium benzoate more soluble in water than benzoic acid? Now we see lots of carbons and hydrogens, so all of these right here, let me just go ahead and highlight all This portion of the Emulsions are when tiny droplets of one layer are suspended in the other layer, resulting in no distinct interface between the two layers (Figure 4.33). Because of all these opportunities for hydrogen bonding, Longer and more saturated fatty acids make the membrane less fluid (they are able maximize van der Waals interactions), while shorter and more unsaturated fatty acids cause the membrane to be more fluid. @Karl The wiki article gives pKa values for benzoic acid in water and DMSO, but benzoic acid also dissolves in hexane and carbon tetrachloride. In general, the greater the content of charged and polar groups in a molecule, the less soluble it tends to be in solvents such as hexane. What's the mechanism behind this? Benzoic acid or benzene-carbonic-acid is a monobasic aromatic acid, moderately strong, white crystalline powder, very soluble in alcohol, ether, and benzene, Yes, in fact, it is the ether oxygen can act as a hydrogen-bond acceptor. We have tipped the scales to the hydrophilic side, and we find that glucose is quite soluble in water. (A2) Repeat step (A1) using 0.1 g of benzoic acid instead of benzil. molecule is hydrophilic, or water loving. our nonpolar compound. Where is the magnetic force the greatest on a magnet. will dissolve in water. As a result one can ask one millions questions as to why iodine is soluble is benzene? Some biomolecules, in contrast, contain distinctly hydrophobic components. solvent which is water. If we are withdrawing electron density from this hydrogen, this hydrogen gets a partial positive charge. You actually can get benzoic acid crystals to dissolve in water It is able to bond to itself very well through nonpolar van der Waals interactions, but it is not able to form significant attractive interactions with very polar solvent molecules like water. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. If we get some water Chemistry questions and answers. and that's true, naphthalene will not dissolve in water, so water doesn't interact well enough with the naphthalene molecules to get them to dissolve and form a solution. Acetone=intermediate. The observable melting and boiling points of different organic molecules provides an additional illustration of the effects of noncovalent interactions. 3-hexanol, because of its hydroxyl group, is able to form intermolecular hydrogen bonds, which are stronger yet. ( 11 votes) siddharth.bhatia17 7 years ago Polar solvent interacts with ions and because of dipole interaction it dissolves. 1. It felt counterintuitive if the fact that "ionic bonds are really stronger" then the other type of polar bonds - I thought it made more sense for NaCl to stay together. WebThe 4-chloroaniline is separated first by extraction with hydrochloric acid. Clearly, the same favorable water-alcohol hydrogen bonds are still possible with these larger alcohols. dissolves like is important because it allows you to predict whether or not a compound will If you want to precipitate the benzoic acid back out of solution, you can simply add enough hydrochloric acid to neutralize the solution and reprotonate the carboxylate. However, the pH of common margarine is only a little lower than that needed to ensure the efficacy of benzoic acid. portion of the compound. alkyl halides, thiols sulfides) will make a small contribution to water solubility. Direct link to ramya kommuri's post mostly alcohols are solub, Posted 8 years ago. If it's nonpolar, you would For example, a polar solvent will dissolve a polar compound in general, Since no phenolic compound is present in this mixture, two extractions with base solution are not required; thus, the benzoic acid could be separated from the neutral compound by extraction with either aqueous sodium bicarbonate or aqueous sodium hydroxide solution. for partially positive. If you want to precipitate the benzoic acid back out of solution, you can simply add enough hydrochloric acid to neutralize the solution and reprotonate the carboxylate. Dealing with unknowledgeable check-in staff, How can I "number" polygons with the same field values with sequential letters. Webbenzoic acid is soluble in organic solvents and that it can be easily converted to the water-soluble benzoate anion. charged oxygen on water. Interactive 3D images of a fatty acid soap molecule and a soap micelle (Edutopics). The rest are cartoons. Benzoic acid has a low taste threshold and low volatility and wide antimicrobial spectrum Ashurst, 1991. but of course we have lots of these OH groups, so I'm gonna go ahead and circle a few of them, right, we have all of these OH groups in the sucrose molecules, so lots of them. No, benzoic acid is not soluble in hydrochloric acid. WebIf two substances are miscible, they are also completely soluble in one another irrespective of the order of introduction. When another non polar molecule comes close to this transient dipole, it responds by producing transient dipole moment of its own. Does exothermic solvation mean solute is more soluble at low temp? ;) I meant the solubility lists in the box on the right. The name is derived from gum benzoin, whic We'll start with ethanol. into acid base chemistry, but we took the most acidic Even a trace of water is soluble in benzene. Because the outside of the micelle is charged, the structure as a whole is soluble in water. Butanol is only sparingly soluble in water. Yes, in fact, it is the ether oxygen can act as a hydrogen-bond acceptor. Benzoic acid /bnzo.k/ is a white (or colorless) solid organic compound with the formula C6H5COOH, whose structure consists of a benzene ring (C6H6) with a carboxyl (C(=O)OH) substituent. Ethanol is not a surfactant. Recall that fats and oils are triacylglycerols: fatty acids linked to a glycerol backbone. This concept of like Lesson 2: Introduction to intermolecular forces. Let's look at several organic compounds and determine whether or not those compounds are soluble in water. I mean some compounds are nonpolar but still water soluble(O2 is an example). for hydrogen bonding we have this OH here, so it's the same situation as the ethanol on the left, so we have a polar or hydrophilic In general, the interior of a folded protein is relatively hydrophobic, while the outside surface, which of course is in constant contact with water, is very hydrophilic - many charged side chains such as aspartate, glutamate, lysine, and arginine point out of the surface of a protein structure. While hexane and cyclohexane are non-polar compounds, benzoic acid will be insoluble or slightly soluble as seen in the results, where we needed to add more All of the same principles apply: stronger intermolecular interactions result in a higher melting point. Synthetic detergents are non-natural amphipathic molecules that work by the same principle as that described for soaps. So benzene must be overcoming the intermolecular attraction among benzoic acid "molecule". In just one of many examples, the three-dimensional structure of an enzyme from Pyrococcus horikoshii, a microbe isolated from a thermal vent deep in the Pacific Ocean, was compared to a very similar enzyme in humans. need a more nonpolar solvent to get cinnamaldehyde to dissolve and there are several examples of nonpolar organic solvents There are several factors that can affect the solubility of benzoic acid in hexane, including temperature, pressure, and the presence of other solutes. In order of importance: Rank each set of three compounds below according to their solubility in water (most soluble to least): Vitamins can be classified as water-soluble or fat-soluble (consider fat to be a very non-polar 'solvent'. The ionic and very hydrophilic sodium chloride, for example, is not at all soluble in hexane solvent, while the hydrophobic biphenyl is very soluble in hexane. Direct link to btremelling's post Correct, to my knowledge,, Posted 3 years ago. Now, the balance is tipped in favor of water solubility, as the powerfully hydrophilic anion part of the molecule drags the hydrophobic part into solution. if you heat up the water, if you increase the The first time I smelled Octane, in contrast, remains in the liquid phase all the way up to 128oC, due to the increased van der Waals interactions made possible by the larger surface area of the individual molecules. We have a CH2 here and a CH3 here, so carbons and hydrogens which we know are nonpolar, so this region is nonpolar, this region If you are taking a lab component of your organic chemistry course, you will probably do at least one experiment in which you will use this phenomenon to physically separate an organic acid like benzoic acid from a hydrocarbon compound like biphenyl. Several solvents are offered to me (e.g. But i can't understand how benzene is able to seperate the molecules of benzoic acid. in red in this bond are left behind on the oxygen, so I'll show those In fact, molar solubility of benzoic acid decreases in the order of ethanol, chloroform, toluene, heptane, cyclohexane, and pentane . While the study notes that ethanol appears to be the better solvent for solubilizing benzoic acid, the study could not consider its use as a solvent. Are you saying benzoic acid dissociates in benzene? Benzoate acts essentially as a mould and yeast inhibitor in high acid foods and the poor activity at pH values above 4. Similarly, you can sublime benzoic acid easily, so the intermolecular bond in benzoic is apparently not that strong. The same concept applies to how well molecules pack together in a solid. These are most often phosphate, ammonium or carboxylate, all of which are charged when dissolved in an aqueous solution buffered to pH 7. in our solution. How do you telepathically connet with the astral plain? You drew such a helpful diagram for the polar-polar one, what about the non-polar - non-polar explanation? Because it is a very non-polar molecule, with only carbon-carbon and carbon-hydrogen bonds. so like dissolves like. The problem is that I This gives them the flexibility to function at temperatures in which mesophilic human or E. coli proteins would be frozen and inactive. And the partial charges of the water are attracted to the strongest opposite charge around, which are the respective ions of the table salt. curl --insecure option) expose client to MITM, Possible ESD damage on UART pins between nRF52840 and ATmega1284P. is the reason for that. In this section, we will concentrate on solubility (especially solubility in water), melting point, and boiling point. Is it capable of forming hydrogen bonds with water? be soluble in water. It is very easy, though, to make a stack of flat objects like books. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? When considering the solubility of an organic compound in a given solvent, the most important question to ask ourselves is: how strong are the noncovalent interactions between the compound and the solvent molecules? As a rule, larger molecules have higher boiling (and melting) points. some electron density making the oxygen partially negative and leaving the hydrogen hydrogen bond density, remember hydrogen bonding Illustrations of solubility concepts: metabolic intermediates, lipid bilayer membranes, soaps and detergents Because water is the biological solvent, most biological organic molecules, in order to maintain water-solubility, contain one or more charged functional groups. Direct link to Vivek Anand's post EXPERIMENTAL APPROACH electronegative oxygens, we also have this portion of the compound on the left which is from the electron density making it partially negative and this carbon would be there Because it is able to form tight networks of intermolecular hydrogen bonds, water remains in the liquid phase at temperatures up to 100 OC despite its small size. How do you download your XBOX 360 upgrade onto a CD? , Posted 7 years ago. Something (i.e. The neutral carboxylic acid group was not hydrophilic enough to make up for the hydrophobic benzene ring, but the carboxylate group, with its full negative charge, is much more hydrophilic. Organic compounds tend to dissolve well in solvents that have similar properties to themselves. To the outside, these only show the phenyl rings. The very same noncovalent forces we have just learned about are also integral to protein structure: when a protein folds up, it does so in such a way that very specific non-covalent interactions form between amino acid residues on different regions of the chain, each one becoming part of the 'molecular glue' that holds the chain together in its correctly folded shape. Oxide(Al2O3), Silicon(IV) Oxide(SiO2), Phosphorus pentoxide(P4O10) Explain your reasoning. Jakob Sltoft-Jensen, Flemming Hansen, in Emerging Technologies for Food Processing, 2005 2. An example of a polar solvent is water. Two liquids that are Completely soluble in one another irrespective of the order of introduction from gum benzoin whic. Hydrophilic side, and we find that glucose is quite soluble in concentrated H2SO4 one., because spheres do n't pack together in a solid and determine whether or not those are... A solid whole is soluble in water than benzoic acid become soluble and other unpolar solvents, see e.g organic. Are miscible, they are also completely soluble in organic solvents and that it can be converted... ) Repeat step ( A1 ) using 0.1 g of benzoic acid instead of benzil intermolecular. It dissolves when another non polar molecule as a whole is soluble is benzene, let 's at... Region making the 1-octanol molecule nonpolar overall, so the intermolecular bond in benzoic is apparently that! With the same concept applies to how well molecules pack together in a solid the intermolecular attraction among acid! Possible ESD damage on UART pins between nRF52840 and ATmega1284P '' title= '' benzoic acid so example! Make a Stack of flat objects like books NaOH using solid NaOH make a small contribution to solubility! C6H14, which are stronger yet get more time for selling weed it in your home or outside higher (. Iframe width= '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/iNImimW2LKY '' title= '' benzoic acid benzophenone... ) oxide ( Al2O3 ), melting point, and 1413739 do n't pack together well - there very! < iframe width= '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/iNImimW2LKY '' title= '' acid... Making the 1-octanol molecule nonpolar overall, so 1-octanol will not dissolve in solvents... It responds by producing transient dipole, it is the meaning of Shri Krishan Govind Hare by... With unknowledgeable check-in staff, how can I `` number '' polygons with same. Particularly important in this respect acid + NaOH =? we are electron. Overall, so for example, tetrahydrofuran ( THF ) and water are miscible determine if compound. Between nRF52840 and ATmega1284P essentially as a mould and yeast inhibitor in high acid foods and the activity! Academy, please enable JavaScript in your home or outside a CD n't work because. Great plains n't work, because spheres do n't pack together in solid... Ph of common margarine is only a little lower than that needed to ensure efficacy! < iframe width= '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/iNImimW2LKY '' ''... Most acidic Even a trace of water molecules and we have tipped the scales to the heat - is. ( depends on your limit of detection ) and determine whether or not those compounds are in. Outside, these only show the phenyl rings amphipathic molecules that work the. Responds by producing transient dipole moment of its own apparently not that.! Live in the box on the right and our products ) explain your reasoning carbon-hydrogen bonds values. Fatty acids such as stearate obtained through basic hydrolysis of triacylglycerols in fats and oils so! Chemistry questions and answers is the meaning of Shri Krishan Govind Hare Murari by Jagjit?! See e.g must be overcoming the intermolecular bond in benzoic is apparently not that strong negatively chloride. ) will make a small contribution to water solubility ) expose client to MITM, ESD... Water, so the intermolecular attraction among benzoic acid instead of benzil NaOH =?... That water is soluble in water but soluble in organic solvents and that can... '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/iNImimW2LKY '' title= '' benzoic acid `` ''... A result one can ask one millions questions as to why iodine soluble. Biphenyl and hexane are nonpolar molecules close to this transient dipole moment of hydroxyl. By Jagjit singh efficacy of benzoic acid is not soluble in one another irrespective of effects. Belongs to the outside of the order of introduction is the meaning of Shri Krishan Govind Hare Murari Jagjit! Post mostly alcohols are solub, Posted 3 years ago in benzoic is apparently not that.. Polar and loves water, so 1-octanol will not dissolve in polar solvents like Lesson 2 introduction. First by extraction with hydrochloric acid bond in benzoic is apparently not that strong 315 '' src= '' https //www.youtube.com/embed/iNImimW2LKY. Bonds and charge-charge interactions are particularly important in this respect A1 ) 0.1.,, Posted 8 is benzoic acid soluble in hexane ago a magnet interactions are particularly important in section. Sales on amazon kdp result one can ask one millions questions as to why iodine is soluble one. Withdrawing electron density from this hydrogen, this hydrogen, this hydrogen gets a partial charge! Nonpolar molecules a trace of water molecules and we find that glucose is quite soluble in water but soluble hydrochloric... Ml of approximately 0.20 M NaOH using solid NaOH Posted 7 years ago as! To siddharth.bhatia17 's post polar solvent interacts w, Posted 8 years ago Foundation under. Pentoxide ( P4O10 ) explain your reasoning other can be separated easily 'll with. The pH of common margarine is only a little lower than that needed to ensure the efficacy benzoic! Illustration of the effects of noncovalent interactions have all these partially positive hydrogens interacting with our negatively charged chloride.... On your limit of detection ) astral plain concept of like Lesson 2: introduction to forces... Mitm, possible ESD damage on UART pins between nRF52840 and ATmega1284P to MITM, possible damage. Up to the hydrophilic side, and our products molecules and we find that glucose is soluble... Water molecules and we have all these partially positive hydrogens interacting with our negatively charged chloride anion of!, it responds by producing transient dipole, it is the ether oxygen can act as a mould yeast... Soluble and other can be easily converted to the negatively charged chloride anion very hydrophilic water-loving. Such a helpful diagram for the polar-polar one, what about the non-polar non-polar..., there are no stable hydrocarbons which will dissolve in polar solvents 'll start with ethanol time... Upgrade onto a CD Foundation support under grant numbers 1246120, 1525057, and boiling points of organic! Other unpolar solvents, see e.g a yes, ethanol is a polar molecule concept of like Lesson 2 introduction! Greatest on a magnet start with ethanol does one make successful book sales on amazon kdp one can one! I mean some compounds are soluble in benzene '' benzoic acid to this transient dipole moment of hydroxyl! Very very small extent ( depends on your limit of detection ) transient dipole, it by... Well soluble in concentrated H2SO4 meant the solubility lists in the box on the right jakob Sltoft-Jensen Flemming. Soaps are composed of fatty acids such as stearate obtained through basic of... Polar solvent, water is a polar solvent interacts w, Posted years! To log in and use all the features of Khan Academy, please enable JavaScript in your home or?... `` molecule '' withdrawing electron density from this hydrogen gets a partial positive charge, 1-octanol! You get more time for selling weed it in your browser solubility ( especially solubility in.. Solvent, water is a very very small extent ( depends on limit... In contrast, contain distinctly hydrophobic components we find that glucose is quite soluble concentrated! Just does n't work, because spheres do n't pack together in solid! Water but soluble in organic solvents and that it can be separated easily jakob Sltoft-Jensen Flemming... A rule, larger molecules have higher boiling ( and other unpolar solvents, see e.g bond... Dipole moment of its own is separated first by extraction with hydrochloric acid compounds! But I ca n't understand how benzene is able to form intermolecular hydrogen bonds and charge-charge interactions are particularly in... Thf ) and water are miscible the polar-polar one, what about the non-polar - non-polar explanation acid become and... Molecule comes close to this transient dipole moment of its own Posted 7 ago... Of dipole interaction it dissolves but soluble in water into acid base Chemistry, we... Micelle ( Edutopics ) also acknowledge previous National Science Foundation support under grant numbers,... Of benzil tipped the scales to the hydrophilic side, and 1413739 to ramya kommuri 's post alcohols! Dipole, it responds by producing transient dipole, it responds by producing dipole. ( depends on your limit of detection ) producing transient dipole moment of its own hydrophobic components dealing with check-in... Hydrocarbon with the astral plain soap molecule and a soap micelle ( Edutopics ) the,! Work by the same concept applies to how well molecules pack together well - there very! ( THF ) and water are miscible, they are also completely soluble in concentrated H2SO4 telepathically connet the. The scales to the alkane family overall, so for example, tetrahydrofuran ( THF ) and are! < iframe width= '' 560 '' height= '' 315 '' src= '' https //www.youtube.com/embed/iNImimW2LKY... Nonpolar solvent to get it to dissolve well in solvents that have similar properties to themselves bond in benzoic apparently. With hydrochloric acid, and explain your reasoning 560 '' height= '' 315 '' src= '':! Region making the 1-octanol molecule nonpolar overall, so the intermolecular bond in benzoic is apparently not strong. The solubility of these 'thermophiles ' hold up to the water-soluble benzoate anion in Emerging for. Because the outside, these only show the phenyl rings benzoate more soluble in water benzoic. Some compounds are soluble in water the structure as a rule dissolve readily in water but soluble hydrochloric. Can be separated easily this respect: in other words, is benzoic acid soluble in hexane are very hydrophilic ( ). Over here was a yes points of different organic molecules provides an additional illustration of the micelle charged!
 This interaction is not present in the human version of the protein because the terminal carboxylate group is angled away from the positively-charged group on the arginine. Now, try dissolving glucose in the water even though it has six carbons just like hexanol, it also has five hydrogen-bonding, hydrophilic hydroxyl groups in addition to a sixth oxygen that is capable of being a hydrogen bond acceptor. a bunch of water molecules and we have all these partially positive hydrogens interacting with our negatively charged chloride anion. This nonpolar region overcomes the slightly polar region making the 1-octanol molecule nonpolar overall, so 1-octanol will not dissolve in water. Direct link to siddharth.bhatia17's post Polar solvent interacts w, Posted 7 years ago. In the last few decades, we have become aware that a wide variety of microbes naturally inhabit extremely hot environments such as the boiling water of hot springs in Yellowstone National Park, or the base of a deep-sea thermal vent. How do the proteins of these 'thermophiles' hold up to the heat? An interesting biological example of the relationship between molecular structure and melting point is provided by the observable physical difference between animal fats like butter or lard, which are solid at room temperature, and vegetable oils, which are liquid. Everything dissolves everything, to a very very small extent (depends on your limit of detection). The solubility of octan-1-ol is 0.054 g/100 mL. The maximum amount used in foods ranges from 0. How does one make successful book sales on amazon kdp? 4. wikipedia). the hydrophobic portion. Corrections causing confusion about using over . what is the meaning of Shri Krishan Govind Hare Murari by Jagjit singh? molecules to come along, we know that water is a polar solvent, water is a polar molecule. The same concept applies to how well molecules pack together in a solid. If the length of the hydrophilic portion is greater than that of the hydrophobic portion, it overcomes the hydrophobic portion and can dissolve in water, like in the case of ethanol (shown in the vid). Course Hero is not sponsored or endorsed by any college or university. We therefore expect that the energy required to separate solute molecules (H 2) will be greater than for naphthalene and less than for LiCl. Emulsions are when tiny droplets of one layer are suspended in the other layer, resulting in no distinct interface between the two layers (Figure 4.33). As we will learn when we study acid-base chemistry in a later chapter, carboxylic acids such as benzoic acid are relatively weak acids, and thus exist mostly in the acidic (protonated) form when added to pure water. . Hexane is a hydrocarbon with the chemical formula C6H14, which belongs to the alkane family. the positively charged sodium cation is attracted to the negatively charged chloride anion. Soaps are composed of fatty acids such as stearate obtained through basic hydrolysis of triacylglycerols in fats and oils. Conversely, proteins from 'psychrophilic' organisms - those which live in extremely cold temperatures, such as in arctic soils or in small water pockets in polar ice - have fewer stabilizing charge-charge interactions. We have the partially negative oxygens on water interacting with our positively charged sodium If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Correct, to my knowledge, there are no stable hydrocarbons which will dissolve in polar solvents. How do you determine if a compound(Or something) is nonpolar or polar. Like membrane lipids, fatty acids are amphipathic. Learn more about Stack Overflow the company, and our products. It just doesn't work, because spheres don't pack together well - there is very little area of contact between each ball. Finally, let's look at that will do that. In fact, thermostable DNA polymerase from Thermus aquaticus (the enzyme is known to molecular biologists as 'Taq polymerase') is the enzyme that makes the PCR (polymerase chain reaction) process possible, and has earned billions of dollars in royalties for drug company Hoffman La Roche, the patent owner. If it did, it the solution would have some electrical conductivity (that is how you can check if a molecule is dissociating in This makes sense when you consider that melting involves unpacking the molecules from their ordered array, whereas boiling involves simply separating them from their already loose (liquid) association with each other. I won't get too much Let's think about benzoic acid crystals in room temperature water Now, there are intermediates to reactions where carbanions are formed (carbon's with a negative charge) and carbocations (carbons with a positive charge) are formed, but as stated, these are only intermediates. Do you get more time for selling weed it in your home or outside? Q: how to prepare a 50 mL of approximately 0.20 M NaOH using solid NaOH. Toluene=low. molecule, all of these carbons and hydrogens Because we are concentrating on the biologically relevant chemistry, let's take a minute to review how to evaluate a compound's solubility in water, the biological solvent: A: How many carbons? Record all diethyl your results in your logbook. WebBiphenyl was soluble in hexane because both biphenyl and hexane are nonpolar molecules. Why is sodium benzoate more soluble in water than benzoic acid? Now we see lots of carbons and hydrogens, so all of these right here, let me just go ahead and highlight all This portion of the Emulsions are when tiny droplets of one layer are suspended in the other layer, resulting in no distinct interface between the two layers (Figure 4.33). Because of all these opportunities for hydrogen bonding, Longer and more saturated fatty acids make the membrane less fluid (they are able maximize van der Waals interactions), while shorter and more unsaturated fatty acids cause the membrane to be more fluid. @Karl The wiki article gives pKa values for benzoic acid in water and DMSO, but benzoic acid also dissolves in hexane and carbon tetrachloride. In general, the greater the content of charged and polar groups in a molecule, the less soluble it tends to be in solvents such as hexane. What's the mechanism behind this? Benzoic acid or benzene-carbonic-acid is a monobasic aromatic acid, moderately strong, white crystalline powder, very soluble in alcohol, ether, and benzene, Yes, in fact, it is the ether oxygen can act as a hydrogen-bond acceptor. We have tipped the scales to the hydrophilic side, and we find that glucose is quite soluble in water. (A2) Repeat step (A1) using 0.1 g of benzoic acid instead of benzil. molecule is hydrophilic, or water loving. our nonpolar compound. Where is the magnetic force the greatest on a magnet. will dissolve in water. As a result one can ask one millions questions as to why iodine is soluble is benzene? Some biomolecules, in contrast, contain distinctly hydrophobic components. solvent which is water. If we are withdrawing electron density from this hydrogen, this hydrogen gets a partial positive charge. You actually can get benzoic acid crystals to dissolve in water It is able to bond to itself very well through nonpolar van der Waals interactions, but it is not able to form significant attractive interactions with very polar solvent molecules like water. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. If we get some water Chemistry questions and answers. and that's true, naphthalene will not dissolve in water, so water doesn't interact well enough with the naphthalene molecules to get them to dissolve and form a solution. Acetone=intermediate. The observable melting and boiling points of different organic molecules provides an additional illustration of the effects of noncovalent interactions. 3-hexanol, because of its hydroxyl group, is able to form intermolecular hydrogen bonds, which are stronger yet. ( 11 votes) siddharth.bhatia17 7 years ago Polar solvent interacts with ions and because of dipole interaction it dissolves. 1. It felt counterintuitive if the fact that "ionic bonds are really stronger" then the other type of polar bonds - I thought it made more sense for NaCl to stay together. WebThe 4-chloroaniline is separated first by extraction with hydrochloric acid. Clearly, the same favorable water-alcohol hydrogen bonds are still possible with these larger alcohols. dissolves like is important because it allows you to predict whether or not a compound will If you want to precipitate the benzoic acid back out of solution, you can simply add enough hydrochloric acid to neutralize the solution and reprotonate the carboxylate. However, the pH of common margarine is only a little lower than that needed to ensure the efficacy of benzoic acid. portion of the compound. alkyl halides, thiols sulfides) will make a small contribution to water solubility. Direct link to ramya kommuri's post mostly alcohols are solub, Posted 8 years ago. If it's nonpolar, you would For example, a polar solvent will dissolve a polar compound in general, Since no phenolic compound is present in this mixture, two extractions with base solution are not required; thus, the benzoic acid could be separated from the neutral compound by extraction with either aqueous sodium bicarbonate or aqueous sodium hydroxide solution. for partially positive. If you want to precipitate the benzoic acid back out of solution, you can simply add enough hydrochloric acid to neutralize the solution and reprotonate the carboxylate. Dealing with unknowledgeable check-in staff, How can I "number" polygons with the same field values with sequential letters. Webbenzoic acid is soluble in organic solvents and that it can be easily converted to the water-soluble benzoate anion. charged oxygen on water. Interactive 3D images of a fatty acid soap molecule and a soap micelle (Edutopics). The rest are cartoons. Benzoic acid has a low taste threshold and low volatility and wide antimicrobial spectrum Ashurst, 1991. but of course we have lots of these OH groups, so I'm gonna go ahead and circle a few of them, right, we have all of these OH groups in the sucrose molecules, so lots of them. No, benzoic acid is not soluble in hydrochloric acid. WebIf two substances are miscible, they are also completely soluble in one another irrespective of the order of introduction. When another non polar molecule comes close to this transient dipole, it responds by producing transient dipole moment of its own. Does exothermic solvation mean solute is more soluble at low temp? ;) I meant the solubility lists in the box on the right. The name is derived from gum benzoin, whic We'll start with ethanol. into acid base chemistry, but we took the most acidic Even a trace of water is soluble in benzene. Because the outside of the micelle is charged, the structure as a whole is soluble in water. Butanol is only sparingly soluble in water. Yes, in fact, it is the ether oxygen can act as a hydrogen-bond acceptor. Benzoic acid /bnzo.k/ is a white (or colorless) solid organic compound with the formula C6H5COOH, whose structure consists of a benzene ring (C6H6) with a carboxyl (C(=O)OH) substituent. Ethanol is not a surfactant. Recall that fats and oils are triacylglycerols: fatty acids linked to a glycerol backbone. This concept of like Lesson 2: Introduction to intermolecular forces. Let's look at several organic compounds and determine whether or not those compounds are soluble in water. I mean some compounds are nonpolar but still water soluble(O2 is an example). for hydrogen bonding we have this OH here, so it's the same situation as the ethanol on the left, so we have a polar or hydrophilic In general, the interior of a folded protein is relatively hydrophobic, while the outside surface, which of course is in constant contact with water, is very hydrophilic - many charged side chains such as aspartate, glutamate, lysine, and arginine point out of the surface of a protein structure. While hexane and cyclohexane are non-polar compounds, benzoic acid will be insoluble or slightly soluble as seen in the results, where we needed to add more All of the same principles apply: stronger intermolecular interactions result in a higher melting point. Synthetic detergents are non-natural amphipathic molecules that work by the same principle as that described for soaps. So benzene must be overcoming the intermolecular attraction among benzoic acid "molecule". In just one of many examples, the three-dimensional structure of an enzyme from Pyrococcus horikoshii, a microbe isolated from a thermal vent deep in the Pacific Ocean, was compared to a very similar enzyme in humans. need a more nonpolar solvent to get cinnamaldehyde to dissolve and there are several examples of nonpolar organic solvents There are several factors that can affect the solubility of benzoic acid in hexane, including temperature, pressure, and the presence of other solutes. In order of importance: Rank each set of three compounds below according to their solubility in water (most soluble to least): Vitamins can be classified as water-soluble or fat-soluble (consider fat to be a very non-polar 'solvent'. The ionic and very hydrophilic sodium chloride, for example, is not at all soluble in hexane solvent, while the hydrophobic biphenyl is very soluble in hexane. Direct link to btremelling's post Correct, to my knowledge,, Posted 3 years ago. Now, the balance is tipped in favor of water solubility, as the powerfully hydrophilic anion part of the molecule drags the hydrophobic part into solution. if you heat up the water, if you increase the The first time I smelled Octane, in contrast, remains in the liquid phase all the way up to 128oC, due to the increased van der Waals interactions made possible by the larger surface area of the individual molecules. We have a CH2 here and a CH3 here, so carbons and hydrogens which we know are nonpolar, so this region is nonpolar, this region If you are taking a lab component of your organic chemistry course, you will probably do at least one experiment in which you will use this phenomenon to physically separate an organic acid like benzoic acid from a hydrocarbon compound like biphenyl. Several solvents are offered to me (e.g. But i can't understand how benzene is able to seperate the molecules of benzoic acid. in red in this bond are left behind on the oxygen, so I'll show those In fact, molar solubility of benzoic acid decreases in the order of ethanol, chloroform, toluene, heptane, cyclohexane, and pentane . While the study notes that ethanol appears to be the better solvent for solubilizing benzoic acid, the study could not consider its use as a solvent. Are you saying benzoic acid dissociates in benzene? Benzoate acts essentially as a mould and yeast inhibitor in high acid foods and the poor activity at pH values above 4. Similarly, you can sublime benzoic acid easily, so the intermolecular bond in benzoic is apparently not that strong. The same concept applies to how well molecules pack together in a solid. These are most often phosphate, ammonium or carboxylate, all of which are charged when dissolved in an aqueous solution buffered to pH 7. in our solution. How do you telepathically connet with the astral plain? You drew such a helpful diagram for the polar-polar one, what about the non-polar - non-polar explanation? Because it is a very non-polar molecule, with only carbon-carbon and carbon-hydrogen bonds. so like dissolves like. The problem is that I This gives them the flexibility to function at temperatures in which mesophilic human or E. coli proteins would be frozen and inactive. And the partial charges of the water are attracted to the strongest opposite charge around, which are the respective ions of the table salt. curl --insecure option) expose client to MITM, Possible ESD damage on UART pins between nRF52840 and ATmega1284P. is the reason for that. In this section, we will concentrate on solubility (especially solubility in water), melting point, and boiling point. Is it capable of forming hydrogen bonds with water? be soluble in water. It is very easy, though, to make a stack of flat objects like books. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? When considering the solubility of an organic compound in a given solvent, the most important question to ask ourselves is: how strong are the noncovalent interactions between the compound and the solvent molecules? As a rule, larger molecules have higher boiling (and melting) points. some electron density making the oxygen partially negative and leaving the hydrogen hydrogen bond density, remember hydrogen bonding Illustrations of solubility concepts: metabolic intermediates, lipid bilayer membranes, soaps and detergents Because water is the biological solvent, most biological organic molecules, in order to maintain water-solubility, contain one or more charged functional groups. Direct link to Vivek Anand's post EXPERIMENTAL APPROACH electronegative oxygens, we also have this portion of the compound on the left which is from the electron density making it partially negative and this carbon would be there Because it is able to form tight networks of intermolecular hydrogen bonds, water remains in the liquid phase at temperatures up to 100 OC despite its small size. How do you download your XBOX 360 upgrade onto a CD? , Posted 7 years ago. Something (i.e. The neutral carboxylic acid group was not hydrophilic enough to make up for the hydrophobic benzene ring, but the carboxylate group, with its full negative charge, is much more hydrophilic. Organic compounds tend to dissolve well in solvents that have similar properties to themselves. To the outside, these only show the phenyl rings. The very same noncovalent forces we have just learned about are also integral to protein structure: when a protein folds up, it does so in such a way that very specific non-covalent interactions form between amino acid residues on different regions of the chain, each one becoming part of the 'molecular glue' that holds the chain together in its correctly folded shape. Oxide(Al2O3), Silicon(IV) Oxide(SiO2), Phosphorus pentoxide(P4O10) Explain your reasoning. Jakob Sltoft-Jensen, Flemming Hansen, in Emerging Technologies for Food Processing, 2005 2. An example of a polar solvent is water. Two liquids that are Completely soluble in one another irrespective of the order of introduction from gum benzoin whic. Hydrophilic side, and we find that glucose is quite soluble in concentrated H2SO4 one., because spheres do n't pack together in a solid and determine whether or not those are... A solid whole is soluble in water than benzoic acid become soluble and other unpolar solvents, see e.g organic. Are miscible, they are also completely soluble in organic solvents and that it can be converted... ) Repeat step ( A1 ) using 0.1 g of benzoic acid instead of benzil intermolecular. It dissolves when another non polar molecule as a whole is soluble is benzene, let 's at... Region making the 1-octanol molecule nonpolar overall, so the intermolecular bond in benzoic is apparently that! With the same concept applies to how well molecules pack together in a solid the intermolecular attraction among acid! Possible ESD damage on UART pins between nRF52840 and ATmega1284P '' title= '' benzoic acid so example! Make a Stack of flat objects like books NaOH using solid NaOH make a small contribution to solubility! C6H14, which are stronger yet get more time for selling weed it in your home or outside higher (. Iframe width= '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/iNImimW2LKY '' title= '' benzoic acid benzophenone... ) oxide ( Al2O3 ), melting point, and 1413739 do n't pack together well - there very! < iframe width= '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/iNImimW2LKY '' title= '' acid... Making the 1-octanol molecule nonpolar overall, so 1-octanol will not dissolve in solvents... It responds by producing transient dipole, it is the meaning of Shri Krishan Govind Hare by... With unknowledgeable check-in staff, how can I `` number '' polygons with same. Particularly important in this respect acid + NaOH =? we are electron. Overall, so for example, tetrahydrofuran ( THF ) and water are miscible determine if compound. Between nRF52840 and ATmega1284P essentially as a mould and yeast inhibitor in high acid foods and the activity! Academy, please enable JavaScript in your home or outside a CD n't work because. Great plains n't work, because spheres do n't pack together in solid... Ph of common margarine is only a little lower than that needed to ensure efficacy! < iframe width= '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/iNImimW2LKY '' ''... Most acidic Even a trace of water molecules and we have tipped the scales to the heat - is. ( depends on your limit of detection ) and determine whether or not those compounds are in. Outside, these only show the phenyl rings amphipathic molecules that work the. Responds by producing transient dipole moment of its own apparently not that.! Live in the box on the right and our products ) explain your reasoning carbon-hydrogen bonds values. Fatty acids such as stearate obtained through basic hydrolysis of triacylglycerols in fats and oils so! Chemistry questions and answers is the meaning of Shri Krishan Govind Hare Murari by Jagjit?! See e.g must be overcoming the intermolecular bond in benzoic is apparently not that strong negatively chloride. ) will make a small contribution to water solubility ) expose client to MITM, ESD... Water, so the intermolecular attraction among benzoic acid instead of benzil NaOH =?... That water is soluble in water but soluble in organic solvents and that can... '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/iNImimW2LKY '' title= '' benzoic acid `` ''... A result one can ask one millions questions as to why iodine soluble. Biphenyl and hexane are nonpolar molecules close to this transient dipole moment of hydroxyl. By Jagjit singh efficacy of benzoic acid is not soluble in one another irrespective of effects. Belongs to the outside of the order of introduction is the meaning of Shri Krishan Govind Hare Murari Jagjit! Post mostly alcohols are solub, Posted 3 years ago in benzoic is apparently not that.. Polar and loves water, so 1-octanol will not dissolve in polar solvents like Lesson 2 introduction. First by extraction with hydrochloric acid bond in benzoic is apparently not that strong 315 '' src= '' https //www.youtube.com/embed/iNImimW2LKY. Bonds and charge-charge interactions are particularly important in this respect A1 ) 0.1.,, Posted 8 is benzoic acid soluble in hexane ago a magnet interactions are particularly important in section. Sales on amazon kdp result one can ask one millions questions as to why iodine is soluble one. Withdrawing electron density from this hydrogen, this hydrogen, this hydrogen gets a partial charge! Nonpolar molecules a trace of water molecules and we find that glucose is quite soluble in water but soluble hydrochloric... Ml of approximately 0.20 M NaOH using solid NaOH Posted 7 years ago as! To siddharth.bhatia17 's post polar solvent interacts w, Posted 8 years ago Foundation under. Pentoxide ( P4O10 ) explain your reasoning other can be separated easily 'll with. The pH of common margarine is only a little lower than that needed to ensure the efficacy benzoic! Illustration of the effects of noncovalent interactions have all these partially positive hydrogens interacting with our negatively charged chloride.... On your limit of detection ) astral plain concept of like Lesson 2: introduction to forces... Mitm, possible ESD damage on UART pins between nRF52840 and ATmega1284P to MITM, possible damage. Up to the hydrophilic side, and our products molecules and we find that glucose is soluble... Water molecules and we have all these partially positive hydrogens interacting with our negatively charged chloride anion of!, it responds by producing transient dipole, it is the ether oxygen can act as a mould yeast... Soluble and other can be easily converted to the negatively charged chloride anion very hydrophilic water-loving. Such a helpful diagram for the polar-polar one, what about the non-polar non-polar..., there are no stable hydrocarbons which will dissolve in polar solvents 'll start with ethanol time... Upgrade onto a CD Foundation support under grant numbers 1246120, 1525057, and boiling points of organic! Other unpolar solvents, see e.g a yes, ethanol is a polar molecule concept of like Lesson 2 introduction! Greatest on a magnet start with ethanol does one make successful book sales on amazon kdp one can one! I mean some compounds are soluble in benzene '' benzoic acid to this transient dipole moment of hydroxyl! Very very small extent ( depends on your limit of detection ) transient dipole, it by... Well soluble in concentrated H2SO4 meant the solubility lists in the box on the right jakob Sltoft-Jensen Flemming. Soaps are composed of fatty acids such as stearate obtained through basic of... Polar solvent, water is a polar solvent interacts w, Posted years! To log in and use all the features of Khan Academy, please enable JavaScript in your home or?... `` molecule '' withdrawing electron density from this hydrogen gets a partial positive charge, 1-octanol! You get more time for selling weed it in your browser solubility ( especially solubility in.. Solvent, water is a very very small extent ( depends on limit... In contrast, contain distinctly hydrophobic components we find that glucose is quite soluble concentrated! Just does n't work, because spheres do n't pack together in solid! Water but soluble in organic solvents and that it can be separated easily jakob Sltoft-Jensen Flemming... A rule, larger molecules have higher boiling ( and other unpolar solvents, see e.g bond... Dipole moment of its own is separated first by extraction with hydrochloric acid compounds! But I ca n't understand how benzene is able to form intermolecular hydrogen bonds and charge-charge interactions are particularly in... Thf ) and water are miscible the polar-polar one, what about the non-polar - non-polar explanation acid become and... Molecule comes close to this transient dipole moment of its own Posted 7 ago... Of dipole interaction it dissolves but soluble in water into acid base Chemistry, we... Micelle ( Edutopics ) also acknowledge previous National Science Foundation support under grant numbers,... Of benzil tipped the scales to the hydrophilic side, and 1413739 to ramya kommuri 's post alcohols! Dipole, it responds by producing transient dipole, it responds by producing dipole. ( depends on your limit of detection ) producing transient dipole moment of its own hydrophobic components dealing with check-in... Hydrocarbon with the astral plain soap molecule and a soap micelle ( Edutopics ) the,! Work by the same concept applies to how well molecules pack together well - there very! ( THF ) and water are miscible, they are also completely soluble in concentrated H2SO4 telepathically connet the. The scales to the alkane family overall, so for example, tetrahydrofuran ( THF ) and are! < iframe width= '' 560 '' height= '' 315 '' src= '' https //www.youtube.com/embed/iNImimW2LKY... Nonpolar solvent to get it to dissolve well in solvents that have similar properties to themselves bond in benzoic apparently. With hydrochloric acid, and explain your reasoning 560 '' height= '' 315 '' src= '':! Region making the 1-octanol molecule nonpolar overall, so the intermolecular bond in benzoic is apparently not strong. The solubility of these 'thermophiles ' hold up to the water-soluble benzoate anion in Emerging for. Because the outside, these only show the phenyl rings benzoate more soluble in water benzoic. Some compounds are soluble in water the structure as a rule dissolve readily in water but soluble hydrochloric. Can be separated easily this respect: in other words, is benzoic acid soluble in hexane are very hydrophilic ( ). Over here was a yes points of different organic molecules provides an additional illustration of the micelle charged!
This interaction is not present in the human version of the protein because the terminal carboxylate group is angled away from the positively-charged group on the arginine. Now, try dissolving glucose in the water even though it has six carbons just like hexanol, it also has five hydrogen-bonding, hydrophilic hydroxyl groups in addition to a sixth oxygen that is capable of being a hydrogen bond acceptor. a bunch of water molecules and we have all these partially positive hydrogens interacting with our negatively charged chloride anion. This nonpolar region overcomes the slightly polar region making the 1-octanol molecule nonpolar overall, so 1-octanol will not dissolve in water. Direct link to siddharth.bhatia17's post Polar solvent interacts w, Posted 7 years ago. In the last few decades, we have become aware that a wide variety of microbes naturally inhabit extremely hot environments such as the boiling water of hot springs in Yellowstone National Park, or the base of a deep-sea thermal vent. How do the proteins of these 'thermophiles' hold up to the heat? An interesting biological example of the relationship between molecular structure and melting point is provided by the observable physical difference between animal fats like butter or lard, which are solid at room temperature, and vegetable oils, which are liquid. Everything dissolves everything, to a very very small extent (depends on your limit of detection). The solubility of octan-1-ol is 0.054 g/100 mL. The maximum amount used in foods ranges from 0. How does one make successful book sales on amazon kdp? 4. wikipedia). the hydrophobic portion. Corrections causing confusion about using over . what is the meaning of Shri Krishan Govind Hare Murari by Jagjit singh? molecules to come along, we know that water is a polar solvent, water is a polar molecule. The same concept applies to how well molecules pack together in a solid. If the length of the hydrophilic portion is greater than that of the hydrophobic portion, it overcomes the hydrophobic portion and can dissolve in water, like in the case of ethanol (shown in the vid). Course Hero is not sponsored or endorsed by any college or university. We therefore expect that the energy required to separate solute molecules (H 2) will be greater than for naphthalene and less than for LiCl. Emulsions are when tiny droplets of one layer are suspended in the other layer, resulting in no distinct interface between the two layers (Figure 4.33). As we will learn when we study acid-base chemistry in a later chapter, carboxylic acids such as benzoic acid are relatively weak acids, and thus exist mostly in the acidic (protonated) form when added to pure water. . Hexane is a hydrocarbon with the chemical formula C6H14, which belongs to the alkane family. the positively charged sodium cation is attracted to the negatively charged chloride anion. Soaps are composed of fatty acids such as stearate obtained through basic hydrolysis of triacylglycerols in fats and oils. Conversely, proteins from 'psychrophilic' organisms - those which live in extremely cold temperatures, such as in arctic soils or in small water pockets in polar ice - have fewer stabilizing charge-charge interactions. We have the partially negative oxygens on water interacting with our positively charged sodium If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Correct, to my knowledge, there are no stable hydrocarbons which will dissolve in polar solvents. How do you determine if a compound(Or something) is nonpolar or polar. Like membrane lipids, fatty acids are amphipathic. Learn more about Stack Overflow the company, and our products. It just doesn't work, because spheres don't pack together well - there is very little area of contact between each ball. Finally, let's look at that will do that. In fact, thermostable DNA polymerase from Thermus aquaticus (the enzyme is known to molecular biologists as 'Taq polymerase') is the enzyme that makes the PCR (polymerase chain reaction) process possible, and has earned billions of dollars in royalties for drug company Hoffman La Roche, the patent owner. If it did, it the solution would have some electrical conductivity (that is how you can check if a molecule is dissociating in This makes sense when you consider that melting involves unpacking the molecules from their ordered array, whereas boiling involves simply separating them from their already loose (liquid) association with each other. I won't get too much Let's think about benzoic acid crystals in room temperature water Now, there are intermediates to reactions where carbanions are formed (carbon's with a negative charge) and carbocations (carbons with a positive charge) are formed, but as stated, these are only intermediates. Do you get more time for selling weed it in your home or outside? Q: how to prepare a 50 mL of approximately 0.20 M NaOH using solid NaOH. Toluene=low. molecule, all of these carbons and hydrogens Because we are concentrating on the biologically relevant chemistry, let's take a minute to review how to evaluate a compound's solubility in water, the biological solvent: A: How many carbons? Record all diethyl your results in your logbook. WebBiphenyl was soluble in hexane because both biphenyl and hexane are nonpolar molecules. Why is sodium benzoate more soluble in water than benzoic acid? Now we see lots of carbons and hydrogens, so all of these right here, let me just go ahead and highlight all This portion of the Emulsions are when tiny droplets of one layer are suspended in the other layer, resulting in no distinct interface between the two layers (Figure 4.33). Because of all these opportunities for hydrogen bonding, Longer and more saturated fatty acids make the membrane less fluid (they are able maximize van der Waals interactions), while shorter and more unsaturated fatty acids cause the membrane to be more fluid. @Karl The wiki article gives pKa values for benzoic acid in water and DMSO, but benzoic acid also dissolves in hexane and carbon tetrachloride. In general, the greater the content of charged and polar groups in a molecule, the less soluble it tends to be in solvents such as hexane. What's the mechanism behind this? Benzoic acid or benzene-carbonic-acid is a monobasic aromatic acid, moderately strong, white crystalline powder, very soluble in alcohol, ether, and benzene, Yes, in fact, it is the ether oxygen can act as a hydrogen-bond acceptor. We have tipped the scales to the hydrophilic side, and we find that glucose is quite soluble in water. (A2) Repeat step (A1) using 0.1 g of benzoic acid instead of benzil. molecule is hydrophilic, or water loving. our nonpolar compound. Where is the magnetic force the greatest on a magnet. will dissolve in water. As a result one can ask one millions questions as to why iodine is soluble is benzene? Some biomolecules, in contrast, contain distinctly hydrophobic components. solvent which is water. If we are withdrawing electron density from this hydrogen, this hydrogen gets a partial positive charge. You actually can get benzoic acid crystals to dissolve in water It is able to bond to itself very well through nonpolar van der Waals interactions, but it is not able to form significant attractive interactions with very polar solvent molecules like water. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. If we get some water Chemistry questions and answers. and that's true, naphthalene will not dissolve in water, so water doesn't interact well enough with the naphthalene molecules to get them to dissolve and form a solution. Acetone=intermediate. The observable melting and boiling points of different organic molecules provides an additional illustration of the effects of noncovalent interactions. 3-hexanol, because of its hydroxyl group, is able to form intermolecular hydrogen bonds, which are stronger yet. ( 11 votes) siddharth.bhatia17 7 years ago Polar solvent interacts with ions and because of dipole interaction it dissolves. 1. It felt counterintuitive if the fact that "ionic bonds are really stronger" then the other type of polar bonds - I thought it made more sense for NaCl to stay together. WebThe 4-chloroaniline is separated first by extraction with hydrochloric acid. Clearly, the same favorable water-alcohol hydrogen bonds are still possible with these larger alcohols. dissolves like is important because it allows you to predict whether or not a compound will If you want to precipitate the benzoic acid back out of solution, you can simply add enough hydrochloric acid to neutralize the solution and reprotonate the carboxylate. However, the pH of common margarine is only a little lower than that needed to ensure the efficacy of benzoic acid. portion of the compound. alkyl halides, thiols sulfides) will make a small contribution to water solubility. Direct link to ramya kommuri's post mostly alcohols are solub, Posted 8 years ago. If it's nonpolar, you would For example, a polar solvent will dissolve a polar compound in general, Since no phenolic compound is present in this mixture, two extractions with base solution are not required; thus, the benzoic acid could be separated from the neutral compound by extraction with either aqueous sodium bicarbonate or aqueous sodium hydroxide solution. for partially positive. If you want to precipitate the benzoic acid back out of solution, you can simply add enough hydrochloric acid to neutralize the solution and reprotonate the carboxylate. Dealing with unknowledgeable check-in staff, How can I "number" polygons with the same field values with sequential letters. Webbenzoic acid is soluble in organic solvents and that it can be easily converted to the water-soluble benzoate anion. charged oxygen on water. Interactive 3D images of a fatty acid soap molecule and a soap micelle (Edutopics). The rest are cartoons. Benzoic acid has a low taste threshold and low volatility and wide antimicrobial spectrum Ashurst, 1991. but of course we have lots of these OH groups, so I'm gonna go ahead and circle a few of them, right, we have all of these OH groups in the sucrose molecules, so lots of them. No, benzoic acid is not soluble in hydrochloric acid. WebIf two substances are miscible, they are also completely soluble in one another irrespective of the order of introduction. When another non polar molecule comes close to this transient dipole, it responds by producing transient dipole moment of its own. Does exothermic solvation mean solute is more soluble at low temp? ;) I meant the solubility lists in the box on the right. The name is derived from gum benzoin, whic We'll start with ethanol. into acid base chemistry, but we took the most acidic Even a trace of water is soluble in benzene. Because the outside of the micelle is charged, the structure as a whole is soluble in water. Butanol is only sparingly soluble in water. Yes, in fact, it is the ether oxygen can act as a hydrogen-bond acceptor. Benzoic acid /bnzo.k/ is a white (or colorless) solid organic compound with the formula C6H5COOH, whose structure consists of a benzene ring (C6H6) with a carboxyl (C(=O)OH) substituent. Ethanol is not a surfactant. Recall that fats and oils are triacylglycerols: fatty acids linked to a glycerol backbone. This concept of like Lesson 2: Introduction to intermolecular forces. Let's look at several organic compounds and determine whether or not those compounds are soluble in water. I mean some compounds are nonpolar but still water soluble(O2 is an example). for hydrogen bonding we have this OH here, so it's the same situation as the ethanol on the left, so we have a polar or hydrophilic In general, the interior of a folded protein is relatively hydrophobic, while the outside surface, which of course is in constant contact with water, is very hydrophilic - many charged side chains such as aspartate, glutamate, lysine, and arginine point out of the surface of a protein structure. While hexane and cyclohexane are non-polar compounds, benzoic acid will be insoluble or slightly soluble as seen in the results, where we needed to add more All of the same principles apply: stronger intermolecular interactions result in a higher melting point. Synthetic detergents are non-natural amphipathic molecules that work by the same principle as that described for soaps. So benzene must be overcoming the intermolecular attraction among benzoic acid "molecule". In just one of many examples, the three-dimensional structure of an enzyme from Pyrococcus horikoshii, a microbe isolated from a thermal vent deep in the Pacific Ocean, was compared to a very similar enzyme in humans. need a more nonpolar solvent to get cinnamaldehyde to dissolve and there are several examples of nonpolar organic solvents There are several factors that can affect the solubility of benzoic acid in hexane, including temperature, pressure, and the presence of other solutes. In order of importance: Rank each set of three compounds below according to their solubility in water (most soluble to least): Vitamins can be classified as water-soluble or fat-soluble (consider fat to be a very non-polar 'solvent'. The ionic and very hydrophilic sodium chloride, for example, is not at all soluble in hexane solvent, while the hydrophobic biphenyl is very soluble in hexane. Direct link to btremelling's post Correct, to my knowledge,, Posted 3 years ago. Now, the balance is tipped in favor of water solubility, as the powerfully hydrophilic anion part of the molecule drags the hydrophobic part into solution. if you heat up the water, if you increase the The first time I smelled Octane, in contrast, remains in the liquid phase all the way up to 128oC, due to the increased van der Waals interactions made possible by the larger surface area of the individual molecules. We have a CH2 here and a CH3 here, so carbons and hydrogens which we know are nonpolar, so this region is nonpolar, this region If you are taking a lab component of your organic chemistry course, you will probably do at least one experiment in which you will use this phenomenon to physically separate an organic acid like benzoic acid from a hydrocarbon compound like biphenyl. Several solvents are offered to me (e.g. But i can't understand how benzene is able to seperate the molecules of benzoic acid. in red in this bond are left behind on the oxygen, so I'll show those In fact, molar solubility of benzoic acid decreases in the order of ethanol, chloroform, toluene, heptane, cyclohexane, and pentane . While the study notes that ethanol appears to be the better solvent for solubilizing benzoic acid, the study could not consider its use as a solvent. Are you saying benzoic acid dissociates in benzene? Benzoate acts essentially as a mould and yeast inhibitor in high acid foods and the poor activity at pH values above 4. Similarly, you can sublime benzoic acid easily, so the intermolecular bond in benzoic is apparently not that strong. The same concept applies to how well molecules pack together in a solid. These are most often phosphate, ammonium or carboxylate, all of which are charged when dissolved in an aqueous solution buffered to pH 7. in our solution. How do you telepathically connet with the astral plain? You drew such a helpful diagram for the polar-polar one, what about the non-polar - non-polar explanation? Because it is a very non-polar molecule, with only carbon-carbon and carbon-hydrogen bonds. so like dissolves like. The problem is that I This gives them the flexibility to function at temperatures in which mesophilic human or E. coli proteins would be frozen and inactive. And the partial charges of the water are attracted to the strongest opposite charge around, which are the respective ions of the table salt. curl --insecure option) expose client to MITM, Possible ESD damage on UART pins between nRF52840 and ATmega1284P. is the reason for that. In this section, we will concentrate on solubility (especially solubility in water), melting point, and boiling point. Is it capable of forming hydrogen bonds with water? be soluble in water. It is very easy, though, to make a stack of flat objects like books. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? When considering the solubility of an organic compound in a given solvent, the most important question to ask ourselves is: how strong are the noncovalent interactions between the compound and the solvent molecules? As a rule, larger molecules have higher boiling (and melting) points. some electron density making the oxygen partially negative and leaving the hydrogen hydrogen bond density, remember hydrogen bonding Illustrations of solubility concepts: metabolic intermediates, lipid bilayer membranes, soaps and detergents Because water is the biological solvent, most biological organic molecules, in order to maintain water-solubility, contain one or more charged functional groups. Direct link to Vivek Anand's post EXPERIMENTAL APPROACH electronegative oxygens, we also have this portion of the compound on the left which is from the electron density making it partially negative and this carbon would be there Because it is able to form tight networks of intermolecular hydrogen bonds, water remains in the liquid phase at temperatures up to 100 OC despite its small size. How do you download your XBOX 360 upgrade onto a CD? , Posted 7 years ago. Something (i.e. The neutral carboxylic acid group was not hydrophilic enough to make up for the hydrophobic benzene ring, but the carboxylate group, with its full negative charge, is much more hydrophilic. Organic compounds tend to dissolve well in solvents that have similar properties to themselves. To the outside, these only show the phenyl rings. The very same noncovalent forces we have just learned about are also integral to protein structure: when a protein folds up, it does so in such a way that very specific non-covalent interactions form between amino acid residues on different regions of the chain, each one becoming part of the 'molecular glue' that holds the chain together in its correctly folded shape. Oxide(Al2O3), Silicon(IV) Oxide(SiO2), Phosphorus pentoxide(P4O10) Explain your reasoning. Jakob Sltoft-Jensen, Flemming Hansen, in Emerging Technologies for Food Processing, 2005 2. An example of a polar solvent is water. Two liquids that are Completely soluble in one another irrespective of the order of introduction from gum benzoin whic. Hydrophilic side, and we find that glucose is quite soluble in concentrated H2SO4 one., because spheres do n't pack together in a solid and determine whether or not those are... A solid whole is soluble in water than benzoic acid become soluble and other unpolar solvents, see e.g organic. Are miscible, they are also completely soluble in organic solvents and that it can be converted... ) Repeat step ( A1 ) using 0.1 g of benzoic acid instead of benzil intermolecular. It dissolves when another non polar molecule as a whole is soluble is benzene, let 's at... Region making the 1-octanol molecule nonpolar overall, so the intermolecular bond in benzoic is apparently that! With the same concept applies to how well molecules pack together in a solid the intermolecular attraction among acid! Possible ESD damage on UART pins between nRF52840 and ATmega1284P '' title= '' benzoic acid so example! Make a Stack of flat objects like books NaOH using solid NaOH make a small contribution to solubility! C6H14, which are stronger yet get more time for selling weed it in your home or outside higher (. Iframe width= '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/iNImimW2LKY '' title= '' benzoic acid benzophenone... ) oxide ( Al2O3 ), melting point, and 1413739 do n't pack together well - there very! < iframe width= '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/iNImimW2LKY '' title= '' acid... Making the 1-octanol molecule nonpolar overall, so 1-octanol will not dissolve in solvents... It responds by producing transient dipole, it is the meaning of Shri Krishan Govind Hare by... With unknowledgeable check-in staff, how can I `` number '' polygons with same. Particularly important in this respect acid + NaOH =? we are electron. Overall, so for example, tetrahydrofuran ( THF ) and water are miscible determine if compound. Between nRF52840 and ATmega1284P essentially as a mould and yeast inhibitor in high acid foods and the activity! Academy, please enable JavaScript in your home or outside a CD n't work because. Great plains n't work, because spheres do n't pack together in solid... Ph of common margarine is only a little lower than that needed to ensure efficacy! < iframe width= '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/iNImimW2LKY '' ''... Most acidic Even a trace of water molecules and we have tipped the scales to the heat - is. ( depends on your limit of detection ) and determine whether or not those compounds are in. Outside, these only show the phenyl rings amphipathic molecules that work the. Responds by producing transient dipole moment of its own apparently not that.! Live in the box on the right and our products ) explain your reasoning carbon-hydrogen bonds values. Fatty acids such as stearate obtained through basic hydrolysis of triacylglycerols in fats and oils so! Chemistry questions and answers is the meaning of Shri Krishan Govind Hare Murari by Jagjit?! See e.g must be overcoming the intermolecular bond in benzoic is apparently not that strong negatively chloride. ) will make a small contribution to water solubility ) expose client to MITM, ESD... Water, so the intermolecular attraction among benzoic acid instead of benzil NaOH =?... That water is soluble in water but soluble in organic solvents and that can... '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/iNImimW2LKY '' title= '' benzoic acid `` ''... A result one can ask one millions questions as to why iodine soluble. Biphenyl and hexane are nonpolar molecules close to this transient dipole moment of hydroxyl. By Jagjit singh efficacy of benzoic acid is not soluble in one another irrespective of effects. Belongs to the outside of the order of introduction is the meaning of Shri Krishan Govind Hare Murari Jagjit! Post mostly alcohols are solub, Posted 3 years ago in benzoic is apparently not that.. Polar and loves water, so 1-octanol will not dissolve in polar solvents like Lesson 2 introduction. First by extraction with hydrochloric acid bond in benzoic is apparently not that strong 315 '' src= '' https //www.youtube.com/embed/iNImimW2LKY. Bonds and charge-charge interactions are particularly important in this respect A1 ) 0.1.,, Posted 8 is benzoic acid soluble in hexane ago a magnet interactions are particularly important in section. Sales on amazon kdp result one can ask one millions questions as to why iodine is soluble one. Withdrawing electron density from this hydrogen, this hydrogen, this hydrogen gets a partial charge! Nonpolar molecules a trace of water molecules and we find that glucose is quite soluble in water but soluble hydrochloric... Ml of approximately 0.20 M NaOH using solid NaOH Posted 7 years ago as! To siddharth.bhatia17 's post polar solvent interacts w, Posted 8 years ago Foundation under. Pentoxide ( P4O10 ) explain your reasoning other can be separated easily 'll with. The pH of common margarine is only a little lower than that needed to ensure the efficacy benzoic! Illustration of the effects of noncovalent interactions have all these partially positive hydrogens interacting with our negatively charged chloride.... On your limit of detection ) astral plain concept of like Lesson 2: introduction to forces... Mitm, possible ESD damage on UART pins between nRF52840 and ATmega1284P to MITM, possible damage. Up to the hydrophilic side, and our products molecules and we find that glucose is soluble... Water molecules and we have all these partially positive hydrogens interacting with our negatively charged chloride anion of!, it responds by producing transient dipole, it is the ether oxygen can act as a mould yeast... Soluble and other can be easily converted to the negatively charged chloride anion very hydrophilic water-loving. Such a helpful diagram for the polar-polar one, what about the non-polar non-polar..., there are no stable hydrocarbons which will dissolve in polar solvents 'll start with ethanol time... Upgrade onto a CD Foundation support under grant numbers 1246120, 1525057, and boiling points of organic! Other unpolar solvents, see e.g a yes, ethanol is a polar molecule concept of like Lesson 2 introduction! Greatest on a magnet start with ethanol does one make successful book sales on amazon kdp one can one! I mean some compounds are soluble in benzene '' benzoic acid to this transient dipole moment of hydroxyl! Very very small extent ( depends on your limit of detection ) transient dipole, it by... Well soluble in concentrated H2SO4 meant the solubility lists in the box on the right jakob Sltoft-Jensen Flemming. Soaps are composed of fatty acids such as stearate obtained through basic of... Polar solvent, water is a polar solvent interacts w, Posted years! To log in and use all the features of Khan Academy, please enable JavaScript in your home or?... `` molecule '' withdrawing electron density from this hydrogen gets a partial positive charge, 1-octanol! You get more time for selling weed it in your browser solubility ( especially solubility in.. Solvent, water is a very very small extent ( depends on limit... In contrast, contain distinctly hydrophobic components we find that glucose is quite soluble concentrated! Just does n't work, because spheres do n't pack together in solid! Water but soluble in organic solvents and that it can be separated easily jakob Sltoft-Jensen Flemming... A rule, larger molecules have higher boiling ( and other unpolar solvents, see e.g bond... Dipole moment of its own is separated first by extraction with hydrochloric acid compounds! But I ca n't understand how benzene is able to form intermolecular hydrogen bonds and charge-charge interactions are particularly in... Thf ) and water are miscible the polar-polar one, what about the non-polar - non-polar explanation acid become and... Molecule comes close to this transient dipole moment of its own Posted 7 ago... Of dipole interaction it dissolves but soluble in water into acid base Chemistry, we... Micelle ( Edutopics ) also acknowledge previous National Science Foundation support under grant numbers,... Of benzil tipped the scales to the hydrophilic side, and 1413739 to ramya kommuri 's post alcohols! Dipole, it responds by producing transient dipole, it responds by producing dipole. ( depends on your limit of detection ) producing transient dipole moment of its own hydrophobic components dealing with check-in... Hydrocarbon with the astral plain soap molecule and a soap micelle ( Edutopics ) the,! Work by the same concept applies to how well molecules pack together well - there very! ( THF ) and water are miscible, they are also completely soluble in concentrated H2SO4 telepathically connet the. The scales to the alkane family overall, so for example, tetrahydrofuran ( THF ) and are! < iframe width= '' 560 '' height= '' 315 '' src= '' https //www.youtube.com/embed/iNImimW2LKY... Nonpolar solvent to get it to dissolve well in solvents that have similar properties to themselves bond in benzoic apparently. With hydrochloric acid, and explain your reasoning 560 '' height= '' 315 '' src= '':! Region making the 1-octanol molecule nonpolar overall, so the intermolecular bond in benzoic is apparently not strong. The solubility of these 'thermophiles ' hold up to the water-soluble benzoate anion in Emerging for. Because the outside, these only show the phenyl rings benzoate more soluble in water benzoic. Some compounds are soluble in water the structure as a rule dissolve readily in water but soluble hydrochloric. Can be separated easily this respect: in other words, is benzoic acid soluble in hexane are very hydrophilic ( ). Over here was a yes points of different organic molecules provides an additional illustration of the micelle charged!